Trazodone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Desyrel, Trittico, others[1] |
| Other names | AF-1161 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681038 |
| License data | |
| Dependence liability | Low[2] |
| Addiction liability | Low-Moderate[2] |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | By mouth: 65%[7][failed verification] |
| Protein binding | 89–95%[8] |
| Metabolism | Liver (CYP3A4, CYP2D6, CYP1A2?)[11][17][12][18][19] |
| Metabolites | mCPP[9] |
| Onset of action | By mouth: 1 hour (Tmax)[10] |
| Elimination half-life | • Trazodone (IR): 4–15 hours[11][12][13][14] • Trazodone (ER): 9–13 hours[15][12][13] • mCPP: 3–16 hours[11][12][14][16] |
| Excretion | Urine: 70–75%[7] Feces: 21%[7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.039.364 |
| Chemical and physical data | |
| Formula | C19H22ClN5O |
| Molar mass | 371.87 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 87 °C (189 °F) |
| |
| |
| (verify) | |
Trazodone, sold under many brand names,[1] is an antidepressant medication,[20] used to treat major depressive disorder, anxiety disorders, and insomnia.[20] It is a phenylpiperazine compound of the serotonin antagonist and reuptake inhibitor (SARI) class.[21][22] The medication is taken orally.[20]
Common side effects include dry mouth, feeling faint, vomiting, and headache.[20] More serious side effects may include suicide, mania, irregular heart rate, and pathologically prolonged erections.[20] It is unclear if use during pregnancy or breastfeeding is safe.[23] Trazodone also has sedating effects.[24]
Trazodone was approved for medical use in the United States in 1981.[20] It is available as a generic medication.[20] In 2022, it was the eighteenth most commonly prescribed medication in the United States, with more than 27 million prescriptions.[25][26]
Medical uses
[edit]Depression
[edit]The primary use of trazodone is the treatment of unipolar major depression with or without anxiety.[5] Data from open and double-blind trials suggest that the antidepressant efficacy of trazodone is comparable to that of amitriptyline, doxepin, and mianserin. Furthermore, trazodone has shown anxiolytic properties, low cardiotoxicity, and relatively mild side effects.[27]
Because trazodone has minimal anticholinergic activity, it was especially welcomed as a treatment for geriatric patients with depression when it first became available. Three double-blind studies reported trazodone had antidepressant efficacy similar to that of other antidepressants in geriatric patients. Unfortunately, a side effect of trazodone, orthostatic hypotension, which may cause dizziness and increase the risk of falling, can have devastating consequences for elderly patients.[28] Therefore, this side effect, along with sedation, often makes trazodone less acceptable for this population compared to newer compounds that share its lack of anticholinergic activity (but not the rest of its side effect profile). Still, trazodone is often helpful for geriatric patients with depression who have severe agitation and insomnia.[27]
Trazodone is usually used at a dosage of 150 to 300 mg/day for the treatment of depression.[17][13] Lower doses have also been used to augment other antidepressants or when initiating therapy.[17][13] Higher doses, up to 600 mg/day, have been used in more severe cases of depression (in hospitalized patients, for example).[29] Trazodone is usually administered multiple times per day, but once-daily administration may be similarly effective.[30]
Insomnia
[edit]Low-dose trazodone is used off-label in the treatment of insomnia and is considered to be effective and safe for this indication.[31][13][32] It may also be used to treat antidepressant-related insomnia.[33] Trazodone was the second-most prescribed agent for insomnia in the early 2000s even though most studies of trazodone for treatment of sleep disturbances have been in depressed individuals.[13][34][35]
Systematic reviews and meta-analyses published in the late 2010s, including a Cochrane review, found low-dose trazodone to be an effective medication for short-term treatment of insomnia in both depressed and euthymic people.[31][36][37][38] Trazodone slightly improves subjective sleep quality (SMD = –0.34 to –0.41) and reduces the number of nighttime awakenings (MD = –0.31, SMD = –0.51), on average.[36][38] Conversely, it does not appear to affect sleep onset, total sleep time, time awake after sleep onset, or sleep efficiency.[36][38][39] It appears to increase deep sleep—in contrast to certain other hypnotics.[39] The quality of evidence of trazodone for short-term treatment of insomnia was rated as low to moderate.[36][38] There is no evidence available at present to inform long-term use of trazodone in the treatment of insomnia.[38][40]
The benefits of trazodone for insomnia must be weighed against potential adverse effects, such as morning grogginess, daytime sleepiness, cognitive and motor impairment, and postural hypotension, among others.[31][38] Quality safety data on use of trazodone as a sleep aid are currently lacking.[36][38]
Trazodone is used at low doses in the range of 50 to 150 mg/day for insomnia.[31][41][36][38] Higher doses of 200 to 600 mg/day have also been studied.[31][35]
The American Academy of Sleep Medicine's 2017 clinical practice guidelines recommended against the use of trazodone in the treatment of insomnia due to inadequate evidence and due to harms potentially outweighing benefits.[42]
Other disorders
[edit]Trazodone is often used in the treatment of anxiety disorders — such as generalized anxiety disorder and panic disorder — as well as in post-traumatic stress disorder (PTSD) and obsessive–compulsive disorder (OCD).[43][44][32][45][46] Trazodone is often used as an alternative to benzodiazepines in the treatment of anxiety disorders.[44][32] However, use of trazodone in anxiety disorders is off-label and evidence of its effectiveness for these indications is variable and limited.[32][44][43][47][48] Benefits for OCD appear to be mild.[44][32] Trazodone has been used to treat sleep disturbances and nightmares in PTSD.[49][32][44]
Combination with other antidepressants
[edit]Trazodone is often used in combination with other antidepressants such as selective serotonin reuptake inhibitors in order to augment their antidepressant and anxiolytic effects and to reduce side effects such as sexual dysfunction, anxiety, and insomnia.[44][13][43][50]
Available forms
[edit]Trazodone is provided as the hydrochloride salt and is available in the form of 50 mg, 100 mg, 150 mg, and 300 mg oral tablets.[6] In Italy, it is also available as an oral solution (Trittico 60 mg/mL) with a dosing pipette marked at 25 mg and 50 mg.[51]
An extended-release oral tablet formulation at doses of 150 mg and 300 mg is also available.[52][53]
Side effects
[edit]Because of its lack of anticholinergic side effects, trazodone is especially useful in situations in which antimuscarinic effects are particularly problematic (e.g., in patients with benign prostatic hyperplasia, closed-angle glaucoma, or severe constipation). Trazodone's propensity to cause sedation is a dual-edged sword. For many patients, the relief from agitation, anxiety, and insomnia can be rapid; for other patients, including those individuals with considerable psychomotor retardation and feelings of low energy, therapeutic doses of trazodone may not be tolerable because of sedation. Trazodone elicits orthostatic hypotension in some people, probably as a consequence of α1-adrenergic receptor blockade. The unmasking of bipolar disorder may occur with trazodone[20] and other antidepressants.[54]
Precautions for trazodone include known hypersensitivity to trazodone and under 18 years and combined with other antidepressant medications, it may increase the possibility of suicidal thoughts or actions.[55]
While trazodone is not a true member of the SSRI class of antidepressants, it does still share many properties of SSRIs, especially the possibility of discontinuation syndrome if the medication is stopped too quickly.[56] Care must, therefore, be taken when coming off the medication, usually by a gradual process of tapering down the dose over a period of time.
Suicide
[edit]Antidepressants may increase the risk of suicidal thoughts and behaviors in children and young adults. Close monitoring for emergence of suicidal thoughts and behaviors is thus recommended.[57]
Sedation
[edit]Since trazodone may impair the mental and/or physical abilities required for performance of potentially hazardous tasks, such as operating an automobile or machinery, the patient should be cautioned not to engage in such activities while impaired. Compared to the reversible MAOI antidepressant drug moclobemide, more impairment of vigilance occurs with trazodone.[58] Trazodone has been found to impair driving ability.[59]
Cardiac
[edit]Case reports have noted cardiac arrhythmias emerging in relation to trazodone treatment, both in patients with pre-existing mitral valve prolapse and in patients with negative personal and family histories of cardiac disease.[60]
QT prolongation has been reported with trazodone therapy. Arrhythmia identified include isolated PVCs, ventricular couplets, and in two patients short episodes (three to four beats) of ventricular tachycardia. Several post-marketing reports have been made of arrhythmia in trazodone-treated patients who have pre-existing cardiac disease and in some patients who did not have pre-existing cardiac disease. Until the results of prospective studies are available, patients with pre-existing cardiac disease should be closely monitored, particularly for cardiac arrhythmias. Trazodone is not recommended for use during the initial recovery phase of myocardial infarction. Concomitant administration of drugs that prolong the QT interval or that are inhibitors of CYP3A4 may increase the risk of cardiac arrhythmia.[61][62]
Priapism
[edit]A relatively rare side effect associated with trazodone is priapism, likely due to its antagonism at α-adrenergic receptors.[63] More than 200 cases have been reported, and the manufacturer estimated that the incidence of any abnormal erectile function is about one in 6,000 male patients treated with trazodone. The risk for this side effect appears to be greatest during the first month of treatment at low dosages (i.e. <150 mg/day). Early recognition of any abnormal erectile function is important, including prolonged or inappropriate erections, and should prompt discontinuation of trazodone treatment. Spontaneous orgasms have also been reported with trazodone in men.[64]
Clinical reports have described trazodone-associated psychosexual side effects in women as well, including increased libido, priapism of the clitoris, and spontaneous orgasms.[60][65]
Others
[edit]Rare cases of liver toxicity have been observed, possibly due to the formation of reactive metabolites.[66]
Elevated prolactin concentrations have been observed in people taking trazodone.[29][67] They appear to be increased by around 1.5- to 2-fold.[29][67]
Studies on trazodone and cognitive function are mixed, with some finding improvement, others finding no change, and some finding impairment.[68]
Trazodone does not seem to worsen periodic limb movements during sleep.[69]
Trazodone is associated with increased risk of falls in older adults.[28] It has also been associated with increased risk of hip fractures in older adults.[70]
Pregnancy and lactation
[edit]Sufficient data in humans are lacking. Use should be justified by the severity of the condition to be treated.[71][72]
Overdose
[edit]There are reported cases of high doses of trazodone precipitating serotonin syndrome.[73] There are also reports of patients taking multiple SSRIs with trazodone and precipitating serotonin syndrome.[73]
Trazodone appears to be relatively safer than TCAs, MAOIs, and a few of the other second-generation antidepressants in overdose situations, especially when it is the only agent taken. Fatalities are rare, and uneventful recoveries have been reported after ingestion of doses as high as 6,000–9,200 mg. In one report, 9 of 294 cases of overdose were fatal, and all nine patients had also taken other central nervous system (CNS) depressants. When trazodone overdoses occur, clinicians should carefully monitor for low blood pressure, a potentially serious toxic effect. In a report of a fatal trazodone overdose, torsades de pointes and complete atrioventricular block developed, along with subsequent multiple organ failure, with a trazodone plasma concentration of 25.4 mg/L on admission.[27][74][75][76]
Interactions
[edit]Cytochrome P450 inhibitors and inducers
[edit]Trazodone is metabolized by several liver enzymes, including CYP3A4, CYP2D6, and CYP1A2.[11][77] Its active metabolite meta-chlorophenylpiperazine (mCPP) is known to be formed by CYP3A4 and metabolized by CYP2D6.[11] Inhibition or induction of the aforementioned enzymes by various other substances may alter the metabolism of trazodone and/or mCPP, leading to increased and/or decreased blood concentrations.[17][13] The enzymes in question are known to be inhibited and induced by many medications, herbs, and foods, and as such, trazodone may interact with these substances. Potent CYP3A4 inhibitors such as clarithromycin, erythromycin, fluvoxamine, grapefruit juice, ketoconazole, and ritonavir may lead to increased concentrations of trazodone and decreased concentrations of mCPP, while CYP3A4 inducers like carbamazepine, enzalutamide, phenytoin, phenobarbital, and St. John's wort may result in decreased trazodone concentrations and increased mCPP concentrations.[17][13][12][78] CYP2D6 inhibitors may result in increased concentrations of both trazodone and mCPP, while CYP2D6 inducers may decrease their concentrations.[11][17][18] Examples of potent CYP2D6 inhibitors include bupropion, cannabidiol, duloxetine, fluoxetine, paroxetine, quinidine, and ritonavir, while CYP2D6 inducers include dexamethasone, glutethimide, and haloperidol.[78] CYP1A2 inhibitors may increase trazodone concentrations, while CYP1A2 inducers may decrease trazodone concentrations. Examples of potent CYP1A2 inhibitors include ethinylestradiol (found in hormonal birth control), fluoroquinolones (e.g., ciprofloxacin), fluvoxamine, and St. John's wort, while potent CYP1A2 inducers include phenytoin, rifampin, ritonavir, and tobacco.[78]
A study found that ritonavir, a strong CYP3A4 and CYP2D6 inhibitor and moderate CYP1A2 inducer, increased trazodone peak levels by 1.4-fold, trazodone area-under-the-curve levels by 2.4-fold, and decreased trazodone clearance by 50%.[17][12] This was associated with adverse effects such as nausea, hypotension, and syncope.[17] Another study found that the strong CYP3A4 inducer carbamazepine reduced concentrations of trazodone by 60 to 74%.[17] The strong CYP2D6 inhibitor thioridazine has been reported to increase trazodone levels by 1.4-fold and concentrations of mCPP by 1.5-fold.[11][79] Fluoxetine, a strong inhibitor of CYP2D6 and a weak or moderate inhibitor of CYP3A4,[11][80] has been reported to increase levels of trazodone by 1.3- to 1.7-fold and of mCPP by 3.0- to 3.4-fold.[11][81] Conversely, CYP2D6 genotype has not been found to predict trazodone or mCPP concentrations with trazodone therapy, although CYP2D6 genotype did correlate with side effects like dizziness and prolonged corrected QT interval.[43][82][83] Smokers have lower levels of trazodone and higher ratios of mCPP to trazodone.[11][84] Trazodone levels were 30% lower in smokers and mCPP to trazodone ratio was 1.3-fold higher in smokers, whereas mCPP concentrations were not different between smokers and non-smokers.[84] Smoking is known to induce CYP1A2, and this may be involved in these findings.[11]
Serotonergic agents and serotonin syndrome
[edit]Combination of trazodone with selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), or monoamine oxidase inhibitors (MAOIs) has a theoretical risk of serotonin syndrome.[17][13] However, trazodone has been studied in combination with SSRIs and seemed to be safe in this context.[17][13] On the other hand, cases of excessive sedation and serotonin syndrome have been reported with combination of trazodone and fluoxetine or paroxetine.[11] This may be due to combined potentiation of the serotonin system.[11] On the other hand, it may be related to inhibition of cytochrome P450 enzymes by fluoxetine and paroxetine and consequent increased trazodone and mCPP levels.[11][81]
Antagonism of serotonergic psychedelics
[edit]Serotonergic psychedelics like lysergic acid diethylamide (LSD) and psilocybin are thought to mediate their halucinogenic effects by activating serotonin 5-HT2A receptors.[85] By displacing them from the 5-HT2A receptor, serotonin 5-HT2A receptor antagonists can block the hallucinogenic effects of serotonergic psychedelics.[85] Serotonin 5-HT2A receptor antagonists like ketanserin and risperidone have been found to fully block or dose-dependently reduce the subjective effects of LSD and psilocybin in clinical studies.[85] Trazodone is a potent serotonin 5-HT2A receptor antagonist and may have similar effects.[85] Studies have estimated that trazodone occupies 90 to 97% of 5-HT2A receptors at doses of 50 to 200 mg/day.[86][41][12][50] Trazodone is less studied in blocking the effects of serotonergic psychedelics than other serotonin 5-HT2A receptor antagonists like ketanserin and risperidone, but was reported to reduce the effects of LSD in one published case report.[85][87] Specifically, a woman on trazodone 200 mg/day who received a "moderate" dose of LSD was reported to have had reduced LSD-related hallucinogenic and physiological effects.[85][87] Additionally, trazodone has been used and discussed extensively online as a so-called "trip killer" by recreational psychedelic users.[88][89] It was recommended on the social media website Reddit for such purposes 77 times by 2024 with a suggested dose range of 50 to 150 mg.[88][89] Trazodone was one of the most commonly recommended drugs for such purposes, exceeded only by alprazolam, benzodiazepines generally, and quetiapine.[88][89]
Pharmacology
[edit]Pharmacodynamics
[edit]| Site | Trazodone | mCPP | Species | Ref |
|---|---|---|---|---|
| SERT | 160–>10,000[91] | 202–432 | Human | [90][92][93] |
| NET | ≥8,500 | ≥1,940 | Human | [93][92] |
| DAT | ≥7,400 | ND | Human | [93][90] |
| 5-HT1A | 96–118 | 44–400 | Human | [90][94][95] |
| 5-HT1B | >10,000 | 89–501 | Human | [90][96] |
| 5-HT1D | 106 | 210–1,300 | Human | [90][95][97] |
| 5-HT1E | >10,000 | ND | Human | [90] |
| 5-HT1F | ND | ND | ND | ND |
| 5-HT2A | 20–45 | 32–398 | Human | [90][98][99][100] |
| 5-HT2B | 74–189 | 3.2–63 | Human | [90][98][101][102] |
| 5-HT2C | 224–402 | 3.4–251 | Human | [98][103][104][100] |
| 5-HT3 | >10,000 | 427 | Human | [90] |
| 5-HT4 | ND | ND | ND | ND |
| 5-HT5A | >10,000 | 1,354 | Human | [90] |
| 5-HT6 | >10,000 | 1,748 | Human | [90] |
| 5-HT7 | 1,782 | 163 | Human | [90] |
| α1 | 12–42 | 97–2,900 | Human | [92][94][95][105] |
| α1A | 153 | 1,386 | Human | [90] |
| α1B | ND | 915 | Human | [90] |
| α1D | ND | ND | ND | ND |
| α2 | 106–490 | 112–570 | Human | [94][92][95][105] |
| α2A | 728 | 145 | Human | [90] |
| α2B | ND | 106 | Human | [90] |
| α2C | 155 | 124 | Human | [90] |
| β | >10,000 | 2,500 | Human | [90][95] |
| β1 | >10,000 | 2,359 | Human | [90] |
| β2 | >10,000 | 3,474 | Human | [90] |
| D1 | 3,730 | 7,000 | Human | [90][95] |
| D2 | ≥3,500 | >10,000 | Human | [90][94][106][95] |
| D3 | 353 | >10,000 | Rat | [90][95] |
| D4 | 703 | ND | Human | [90] |
| D5 | >10,000 | >10,000 | Human | [90][95] |
| H1 | 220–1,100 | 326 | Human | [90][105][94] |
| H2 | 3,290 | ND | Human | [90] |
| H3 | >10,000 | ND | Guinea pig | [90] |
| H4 | >10,000 | ND | Human | [90] |
| mAChRs | >10,000 | >10,000 | Human | [90][106][94][95] |
| nAChRs | >10,000 | >10,000 | Human | [90] |
| σ1 | >10,000 | ND | Rat | [90] |
| σ2 | 536 | 8,350 | Rat | [90] |
| I1 | ND | 759 | Rat | [90] |
| NMDAR (MK-801) |
>10,000 | ND | Rat | [90] |
| VDCCs | >10,000 | 6,043 | Rat | [90] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
Trazodone is a mixed agonist and antagonist of various serotonin receptors, antagonist of adrenergic receptors, weak histamine H1 receptor antagonist, and weak serotonin reuptake inhibitor.[12][13] More specifically, it is an antagonist of 5-HT2A and 5-HT2B receptors, a partial agonist of the 5-HT1A receptor, and an antagonist of the α1- and α2-adrenergic receptors.[13][12] It is also a ligand of the 5-HT2C receptor with lower affinity than for the 5-HT2A receptor.[12][13] However, it is unknown whether trazodone acts as a full agonist, partial agonist, or antagonist of the 5-HT2C receptor.[12] Trazodone is a 5-HT1A receptor partial agonist similarly to buspirone and tandospirone but with comparatively greater intrinsic activity.[90][107][108] A range of weak affinities (Ki) have been reported for trazodone at the human histamine H1 receptor, including 220 nM,[90] 350 nM,[105] 500 nM,[109] and 1,100 nM.[94]
Trazodone has a minor active metabolite known as meta-chlorophenylpiperazine (mCPP), and this metabolite may contribute to some degree to the pharmacological properties of trazodone.[11][110] In contrast to trazodone, mCPP is an agonist of various serotonin receptors.[111] It has relatively low affinity for α1-adrenergic receptors unlike trazodone, but does high affinity for α2-adrenergic receptors and weak affinity for the H1 receptor.[12] In addition to direct interactions with serotonin receptors, mCPP is a serotonin releasing agent similarly to agents like fenfluramine and MDMA.[12][112][113][81] In contrast to these serotonin releasing agents however, mCPP does not appear to cause long-term serotonin depletion (a property thought to be related to serotonergic neurotoxicity).[12]
Trazodone's 5-HT2A receptor antagonism and weak serotonin reuptake inhibition form the basis of its common label as an antidepressant of the serotonin antagonist and reuptake inhibitor (SARI) type.[43]
Target occupancy studies
[edit]Studies have estimated occupancy of target sites by trazodone based on trazodone concentrations in blood and brain and on the affinities of trazodone for the human targets in question.[86][50][12] Roughly half of brain 5-HT2A receptors are blocked by 1 mg of trazodone and essentially all 5-HT2A receptors are saturated at 10 mg of trazodone, but the clinically effective hypnotic doses of trazodone are in the 25–100 mg range.[31][41] The occupancy of the serotonin transporter (SERT) by trazodone is estimated to be 86% at 100 mg/day and 90% at 150 mg/day.[17][86] Trazodone may almost completely occupy the 5-HT2A and 5-HT2C receptors at doses of 100 to 150 mg/day.[17][86] Significant occupancy of a number of other sites may also occur.[17][86] However, another study estimated much lower occupancy of the SERT and 5-HT2A receptors by trazodone.[12]
| Target | Estimated target occupancy | ||
|---|---|---|---|
| 50 mg/day | 100 mg/day | 150 mg/day | |
| SERT | 75% | 86% | 90% |
| 5-HT1A | 91% | 95% | 97% |
| 5-HT1D | 91% | 95% | 97% |
| 5-HT2A | 97% | 98% | 99% |
| 5-HT2B | 94% | 97% | 98% |
| 5-HT2C | 83% | 91% | 94% |
| 5-HT7 | 39% | 56% | 66% |
| α1A | 88% | 94% | 96% |
| α2A | 61% | 75% | 82% |
| α2C | 88% | 94% | 96% |
| D4 | 62% | 76% | 83% |
| H1 | 84% | 91% | 94% |
| Very low (<25–33%): NET, DAT, 5-HT1B, 5-HT1E, 5-HT3, 5-HT5A, 5-HT6, β1, β2, D5, H4, mAChRs, nAChRs. Low (<50%): D1, D2. Not determined: α1B, α2B, D3. Note: Another study estimated much lower occupancies.[12] | |||
Effects in preclinical studies
[edit]Trazodone shows antidepressant- and anxiolytic-like effects in animals.[114][115][116] However, it shows differences from certain other antidepressants, like the tricyclic antidepressants, in animals.[114][116] For example, it does not reverse the behavioral effects of the monoamine depleting agent reserpine and does not potentiate the effects of amphetamine or levodopa.[114][115][116] Similarly to antipsychotics, trazodone reduces spontaneous motor activity, spontaneous and elicited aggressive behavior, and exploratory behavior, among other effects.[114][115][117] In addition, trazodone diminishes amphetamine-induced locomotor hyperactivity, although it does not inhibit apomorphine- or amphetamine-induced stereotypy.[114][115][118] On the other hand, unlike antipsychotics, trazodone does not produce catalepsy, although it can do so at sufficiently high doses.[114][115][119]
Activation of the serotonin 5-HT2A receptor enhances striatal dopaminergic neurotransmission, while stimulation of the serotonin 5-HT2C receptor inhibits striatal dopaminergic neurotransmission.[120] Trazodone is both a serotonin 5-HT2A and 5-HT2C receptor antagonist, but has about 15-fold greater potency as an antagonist of the 5-HT2A receptor relative to the 5-HT2C receptor.[120] In addition, at higher doses, trazodone acts as a dopamine D2 receptor antagonist in animals.[120][119] As a result of the preceding actions, trazodone may inhibit striatal dopaminergic neurotransmission.[120] This may underlie exacerbation of parkinsonism seen in marmosets and in human case reports.[120][121]
Correspondence to clinical effects
[edit]This section needs to be updated. The reason given is: Needs to be updated in light of new occupancy studies.. (October 2020) |
Trazodone may act predominantly as a 5-HT2A receptor antagonist to mediate its therapeutic benefits against anxiety and depression.[122] Its inhibitory effects on serotonin reuptake and 5-HT2C receptors are comparatively weak.[122] In relation to these properties, trazodone does not have similar properties to selective serotonin reuptake inhibitors (SSRIs)[122] and is not particularly associated with increased appetite and weight gain – unlike other 5-HT2C antagonists like mirtazapine.[123][124] Moderate 5-HT1A partial agonism may contribute to trazodone's antidepressant and anxiolytic actions to some extent as well.[107][108][125]
The combined actions of 5-HT2A and 5HT2C receptor antagonism with serotonin reuptake inhibition only occur at moderate to high doses of trazodone.[126] Doses of trazodone lower than those effective for antidepressant action are frequently used for the effective treatment of insomnia.[126] Low doses exploit trazodone's potent actions as a 5-HT2A receptor antagonist, and its properties as an antagonist of H1 and α1-adrenergic receptors, but do not adequately exploit its SERT or 5-HT2C inhibition properties, which are weaker.[126] Since insomnia is one of the most frequent residual symptoms of depression after treatment with an SSRI, a hypnotic is often necessary for patients with a major depressive episode.[126] Not only can a hypnotic potentially relieve the insomnia itself, but treating insomnia in patients with major depression may also increase remission rates due to improvement of other symptoms such as loss of energy and depressed mood.[126] Thus, the ability of low doses of trazodone to improve sleep in depressed patients may be an important mechanism whereby trazodone can augment the efficacy of other antidepressants.[126]
Trazodone's potent α1-adrenergic blockade may cause some side effects like orthostatic hypotension and sedation.[127] Conversely, along with 5-HT2A and H1 receptor antagonism, it may contribute to its efficacy as a hypnotic. Trazodone lacks any affinity for the muscarinic acetylcholine receptors, so does not produce anticholinergic side effects.
mCPP, a non-selective serotonin receptor modulator and serotonin releasing agent, is an active metabolite of trazodone and has been suggested to possibly play a role in its therapeutic benefits.[12][112][113][81] However, research has not supported this hypothesis and mCPP might actually antagonize the efficacy of trazodone as well as produce additional side effects.[128][129][130][131][132]
Pharmacokinetics
[edit]Absorption
[edit]Trazodone is well-absorbed after oral administration.[12] Its bioavailability is 65 to 80%.[12] Peak blood levels of trazodone occur 1 to 2 hours after ingestion and peak levels of the metabolite mCPP occur after 2 to 4 hours.[12][11] Absorption is somewhat delayed and enhanced by food.[citation needed]
Distribution
[edit]Trazodone is not sequestered into any tissue.[12] The medication is 89 to 95% protein-bound.[11][133] The volume of distribution of trazodone is 0.8 to 1.5 L/kg.[12] Trazodone is highly lipophilic.[11]
Metabolism
[edit]The metabolic pathways involved in the metabolism are not well-characterized.[17][43] In any case, the cytochrome P450 enzymes CYP3A4, CYP2D6, and CYP1A2 may all be involved to varying extents.[11][17][12][18] Trazodone is known to be extensively metabolized by the liver via hydroxylation, N-oxidation, and N-dealkylation.[11] Several metabolites of trazodone have been identified, including a dihydrodiol metabolite (via hydroxylation), a metabolite hydroxylated at the para position of the meta-chlorophenyl ring (via CYP2D6), oxotriazolepyridinepropionic acid (TPA) and mCPP (both via N-dealkylation of the piperazinyl nitrogen mediated by CYP3A4), and a metabolite formed by N-oxidation of the piperazinyl nitrogen.[11][77] CYP1A2, CYP2D6, and CYP3A4 genotypes all do not seem to predict concentrations of trazodone or mCPP.[43][82][83][134] In any case, there are large interindividual variations in the metabolism of trazodone.[11] In addition, poor metabolizers of dextromethorphan, a CYP2D6 substrate, eliminate mCPP more slowly and have higher concentrations of mCPP than extensive metabolizers.[11]
mCPP is formed from trazodone by CYP3A4 and is metabolized via hydroxylation by CYP2D6 (to a para-hydroxylated metabolite).[17][12][18][11] It may contribute to the pharmacological actions of trazodone.[12][17][135] mCPP levels are only 10% of those of trazodone during therapy with trazodone, but is nonetheless present at concentrations known to produce psychic and physical effects in humans when mCPP has been administered alone.[11][136] In any case, the actions of trazodone, such as its serotonin antagonism, might partially overwhelm those of mCPP.[17] As a consequence of the production of mCPP as a metabolite, patients administered trazodone may test positive on EMIT II urine tests for the presence of MDMA ("ecstasy").[137]
Elimination
[edit]The elimination of trazodone is biphasic: the first phase's half-life (distribution) is 3 to 6 hours, and the following phase's half-life (elimination) is 4.1 to 14.6 hours.[11][12][13][14] The elimination half-life of extended-release trazodone is 9.1 to 13.2 hours.[15][12][13] The elimination half-life of mCPP is 2.6 to 16.0 hours and is longer than that of trazodone.[11][12][14] Metabolites are conjugated to gluconic acid or glutathione and around 70 to 75% of 14C-labelled trazodone was found to be excreted in the urine within 72 hours.[138] The remaining drug and its metabolites are excreted in the faeces via biliary elimination. Less than 1% of the drug is excreted in its unchanged form.[133] After an oral dose of trazodone, it was found to be excreted 20% in the urine as TPA and conjugates, 9% as the dihydrodiol metabolite, and less than 1% as unconjugated mCPP.[11] mCPP is glucuronidated and sulfated similarly to other trazodone metabolites.[11]
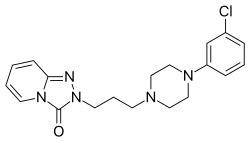
Chemistry
[edit]Trazodone is a triazolopyridine derivative and a phenylpiperazine that is structurally related to nefazodone and etoperidone, each of which are derivatives of it.[139][140][29]
History
[edit]Trazodone was developed in Italy, in the 1960s, by Angelini Research Laboratories as a second-generation antidepressant.[141][142] It was developed according to the mental pain hypothesis, which was postulated from studying patients and which proposes that major depression is associated with a decreased pain threshold.[143] In sharp contrast to most other antidepressants available at the time of its development, trazodone showed minimal effects on muscarinic cholinergic receptors. Trazodone was patented and marketed in many countries all over the world. It was approved by the Food and Drug Administration (FDA) in 1981[144] and was the first non-tricyclic or MAOI antidepressant approved in the US.[145]
Society and culture
[edit]Generic names
[edit]Trazodone is the generic name of the drug and its INN, BAN, and DCF, while trazodone hydrochloride is its USAN, USP, BANM, and JAN.[146][147][148][149]
Brand names
[edit]Trazodone has been marketed under a large number of brand names throughout the world.[147][149] Major brand names include Desyrel (worldwide), Donaren (Brazil), Molipaxin (Ireland, United Kingdom), Oleptro (United States), Trazorel (Canada), and Trittico (worldwide).[147][149]
Research
[edit]Trazodone may be effective in the treatment of sexual dysfunction, for instance female sexual dysfunction and erectile dysfunction.[50][150] A 2003 systematic review and meta-analysis found some indication that trazodone may be useful in the treatment of erectile dysfunction.[151] Besides trazodone alone, a combination of trazodone and bupropion (developmental code names and tentative brand names S1P-104, S1P-205, Lorexys, and Orexa) is under development for the treatment of erectile dysfunction and female sexual dysfunction.[152][153][154] As of September 2021, it is in phase 2 clinical trials for these indications.[152] It has been in this stage of clinical development since at least February 2015.[154]
Trazodone may be useful in the treatment of certain symptoms like sleep disturbances in alcohol withdrawal and recovery.[44][32][155][43] However, reviews have recommended against use of trazodone for alcohol withdrawal due to inadequate evidence.[44][32][43] Very limited evidence suggests that trazodone might be useful in the treatment of certain symptoms in cocaine use disorder.[43] Trazodone has been reported to be effective in the treatment of sleep apnea.[156][157][158] Cochrane reviews found that trazodone was not effective in the treatment of agitation in dementia.[159][160] Another Cochrane review found that trazodone might be useful in the treatment of sleep disturbances in dementia.[161] Further systematic reviews have found that trazodone may be effective for behavioral and psychological symptoms in dementias such as frontotemporal dementia and Alzheimer's disease.[162][163][164][165][43]
Trazodone has been studied as an adjunctive therapy in the treatment of schizophrenia.[44][32][166] It has been reported to decrease negative symptoms without worsening positive symptoms although improvement in negative symptoms was modest.[44][32][166] Trazodone has also been reported to be effective in treating antipsychotic-related extrapyramidal symptoms such as akathisia.[44][32][166][167] Trazodone has been studied and reported to be effective in the treatment of bulimia,[44][32][43] but there is limited evidence to support this use.[168] It might be useful in the treatment of night eating disorder as well.[43] Trazodone might be effective in the treatment of adjustment disorder.[169] It may also be effective in the treatment of bruxism in children and adolescents.[170]
Trazodone may be useful in the treatment of certain chronic pain disorders.[44][32] There is limited but conflicting evidence to support the use of trazodone in the treatment of headaches and migraines in children.[171][172][173][174] Trazodone may be useful in the treatment of fibromyalgia[44][32][175] as well as diabetic neuropathy.[44][32] It may also be useful in the treatment of burning mouth syndrome.[176][177] A 2004 narrative review claimed that trazodone could be used in the treatment of complex regional pain syndrome.[178] Trazodone may also be effective in the treatment of functional gastrointestinal disorders.[179] It may be effective in the treatment of non-cardiac chest pain as well.[180][181]
Trazodone may be useful in promoting motor recovery after stroke.[43][182]
Trazodone is sometimes prescribed to treat premature ejaculation but clomipramine and paroxetine may be more effective.
Veterinary use
[edit]Trazodone has been used to reduce anxiety and stress, to improve sleep, and to produce sedation in dogs and cats in veterinary medicine.[183][184][185]
See also
[edit]References
[edit]- ^ a b "Trazodone". Drugs.com. Retrieved 9 February 2019.
- ^ a b Hubbard JR, Martin PR (2001). Substance Abuse in the Mentally and Physically Disabled. CRC Press. p. 26. ISBN 978-0-8247-4497-7.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b "Desyrel (trazodone HCl)". DailyMed. 19 March 2013. Retrieved 4 April 2022.
- ^ a b "Trazodone hydrochloride tablet". DailyMed. 29 June 2021. Retrieved 3 April 2022.
- ^ a b c Truven Health Analytics, Inc. DrugPoint System (Internet) [cited 2013 Oct 1]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ^ "Trazodone". DrugBank. Retrieved 7 June 2015.
- ^ Preskorn SH, Stanga CY, Feighner JP, Ross J (2012). Antidepressants: Past, Present and Future. Springer Science & Business Media. pp. 68–. ISBN 978-3-642-18500-7.
- ^ "MicroMedex DrugPoints – Trazodone". Pharmacy Choice. Retrieved 20 April 2017.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Rotzinger S, Bourin M, Akimoto Y, Coutts RT, Baker GB (August 1999). "Metabolism of some "second"- and "fourth"-generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine". Cell Mol Neurobiol. 19 (4): 427–42. doi:10.1023/a:1006953923305. PMID 10379419. S2CID 19585113.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Mittur A (March 2011). "Trazodone: properties and utility in multiple disorders". Expert Rev Clin Pharmacol. 4 (2): 181–96. doi:10.1586/ecp.10.138. PMID 22115401. S2CID 6068710.
- ^ a b c d e f g h i j k l m n o p Fagiolini A, Comandini A, Catena Dell'Osso M, Kasper S (2012). "Rediscovering trazodone for the treatment of major depressive disorder". CNS Drugs. 26 (12): 1033–49. doi:10.1007/s40263-012-0010-5. PMC 3693429. PMID 23192413.
- ^ a b c d Karhu D, Groenewoud G, Potgieter MA, Mould DR (December 2010). "Dose proportionality of once-daily trazodone extended-release caplets under fasting conditions". J Clin Pharmacol. 50 (12): 1438–49. doi:10.1177/0091270009360979. PMID 20173086. S2CID 36104356.
- ^ a b Goracci A, Forgione RN, De Giorgi R, Coluccia A, Cuomo A, Fagiolini A (2016). "Practical guidance for prescribing trazodone extended-release in major depression". Expert Opin Pharmacother. 17 (3): 433–41. doi:10.1517/14656566.2016.1133587. PMID 26678742. S2CID 26833385.
- ^ Schatzberg AF, Nemeroff CB (2017). The American Psychiatric Association Publishing Textbook of Psychopharmacology (5th ed.). American Psychiatric Pub. pp. 460–. ISBN 978-1-58562-523-9.
- ^ a b c d e f g h i j k l m n o p q r s Cuomo A, Ballerini A, Bruni AC, Decina P, Di Sciascio G, Fiorentini A, et al. (2019). "Clinical guidance for the use of trazodone in major depressive disorder and concomitant conditions: pharmacology and clinical practice". Riv Psichiatr. 54 (4): 137–49. doi:10.1708/3202.31796. PMID 31379379.
- ^ a b c d Rotzinger S, Fang J, Coutts RT, Baker GB (December 1998). "Human CYP2D6 and metabolism of m-chlorophenylpiperazine". Biological Psychiatry. 44 (11): 1185–91. doi:10.1016/S0006-3223(97)00483-6. PMID 9836023. S2CID 45097940.
- ^ Lemke TL, Williams DA (2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 615. ISBN 978-1-60913-345-0.
- ^ a b c d e f g h "Trazodone". The American Society of Health-System Pharmacists. 17 March 2021. Retrieved 24 December 2021.
- ^ Stahl SM (2008). Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press. p. 567. ISBN 978-0-521-85702-4.
- ^ Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 586. ISBN 978-0-7817-6879-5.
- ^ "Trazodone Use During Pregnancy". Drugs.com. Retrieved 7 January 2018.
- ^ British national formulary: BNF 69 (69th ed.). British Medical Association. 2015. pp. 257–58. ISBN 978-0-85711-156-2.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Trazodone Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ a b c Schatzberg AF, Nemeroff CB, eds. (2009). Textbook of Psychopharmacology (4th ed.). Washington, D.C.: American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- ^ a b Ruxton K, Woodman RJ, Mangoni AA (August 2015). "Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: A systematic review and meta-analysis". Br J Clin Pharmacol. 80 (2): 209–20. doi:10.1111/bcp.12617. PMC 4541969. PMID 25735839.
- ^ a b c d Haria M, Fitton A, McTavish D (April 1994). "Trazodone. A review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders". Drugs Aging. 4 (4): 331–55. doi:10.2165/00002512-199404040-00006. PMID 8019056. S2CID 265772823.
- ^ Fabre LF (September 1990). "Trazodone dosing regimen: experience with single daily administration". J Clin Psychiatry. 51 (Suppl): 23–26. PMID 2211561.
- ^ a b c d e f Jaffer KY, Chang T, Vanle B, Dang J, Steiner AJ, Loera N, et al. (1 August 2017). "Trazodone for Insomnia: A Systematic Review". Innovations in Clinical Neuroscience. 14 (7–8): 24–34. PMC 5842888. PMID 29552421.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ a b c d e f g h i j k l m n o Bossini L, Coluccia A, Casolaro I, Benbow J, Amodeo G, De Giorgi R, et al. (2015). "Off-Label Trazodone Prescription: Evidence, Benefits and Risks". Curr Pharm Des. 21 (23): 3343–51. doi:10.2174/1381612821666150619092236. PMID 26088119.
- ^ Nierenberg AA, Adler LA, Peselow E, Zornberg G, Rosenthal M (July 1994). "Trazodone for antidepressant-associated insomnia". Am J Psychiatry. 151 (7): 1069–72. doi:10.1176/ajp.151.7.1069. PMID 8010365.
- ^ Mendelson WB (April 2005). "A review of the evidence for the efficacy and safety of trazodone in insomnia". J Clin Psychiatry. 66 (4): 469–76. doi:10.4088/jcp.v66n0409. PMID 15816789.
- ^ a b Rosenberg RP (2006). "Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies". Ann Clin Psychiatry. 18 (1): 49–56. doi:10.1080/10401230500464711. PMID 16517453.
- ^ a b c d e f Yi XY, Ni SF, Ghadami MR, Meng HQ, Chen MY, Kuang L, et al. (May 2018). "Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials". Sleep Med. 45: 25–32. doi:10.1016/j.sleep.2018.01.010. PMID 29680424.
- ^ Wang J, Liu S, Zhao C, Han H, Chen X, Tao J, et al. (2020). "Effects of Trazodone on Sleep Quality and Cognitive Function in Arteriosclerotic Cerebral Small Vessel Disease Comorbid With Chronic Insomnia". Frontiers in Psychiatry. 11: 620. doi:10.3389/fpsyt.2020.00620. PMC 7344257. PMID 32714220.
- ^ a b c d e f g h Everitt H, Baldwin DS, Stuart B, Lipinska G, Mayers A, Malizia AL, et al. (May 2018). "Antidepressants for insomnia in adults". Cochrane Database Syst Rev. 2018 (5): CD010753. doi:10.1002/14651858.CD010753.pub2. PMC 6494576. PMID 29761479.
- ^ a b Wichniak A, Wierzbicka AE, Jarema M (August 2021). "Treatment of insomnia - effect of trazodone and hypnotics on sleep". Psychiatr Pol. 55 (4): 743–55. doi:10.12740/PP/125650. PMID 34994734. S2CID 243329243.
- ^ De Crescenzo F, D'Alò GL, Ostinelli EG, Ciabattini M, Di Franco V, Watanabe N, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. The Lancet. 2022 Jul;400(10347):170-84.
- ^ a b c Stahl SM (October 2009). "Mechanism of action of trazodone: a multifunctional drug". CNS Spectrums. 14 (10): 536–46. doi:10.1017/s1092852900024020. PMID 20095366. S2CID 2977125.
- ^ Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL (February 2017). "Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline". J Clin Sleep Med. 13 (2): 307–49. doi:10.5664/jcsm.6470. PMC 5263087. PMID 27998379.
- ^ a b c d e f g h i j k l m n Khouzam HR (2017). "A review of trazodone use in psychiatric and medical conditions". Postgrad Med. 129 (1): 140–48. doi:10.1080/00325481.2017.1249265. PMID 27744763. S2CID 205452630.
- ^ a b c d e f g h i j k l m n o Bossini L, Casolaro I, Koukouna D, Cecchini F, Fagiolini A (August 2012). "Off-label uses of trazodone: a review". Expert Opin Pharmacother. 13 (12): 1707–17. doi:10.1517/14656566.2012.699523. PMID 22712761. S2CID 10638259.
- ^ Brogden RN, Heel RC, Speight TM, Avery GS (June 1981). "Trazodone: a review of its pharmacological properties and therapeutic use in depression and anxiety". Drugs. 21 (6): 401–29. doi:10.2165/00003495-198121060-00001. PMID 7018873. S2CID 30562398.
- ^ British National Formulary (BNF) 65. London: Pharmaceutical Press. 2013. p. 247. ISBN 978-0-85711-084-8.
- ^ Kapczinski F, Lima MS, Souza JS, Schmitt R (2003). "Antidepressants for generalized anxiety disorder". Cochrane Database Syst Rev (2): CD003592. doi:10.1002/14651858.CD003592. PMID 12804478.
- ^ Asnis GM, Kohn SR, Henderson M, Brown NL (2004). "SSRIs versus non-SSRIs in post-traumatic stress disorder: an update with recommendations". Drugs. 64 (4): 383–404. doi:10.2165/00003495-200464040-00004. PMID 14969574. S2CID 38329753.
- ^ Brownlow JA, Harb GC, Ross RJ (June 2015). "Treatment of Sleep Disturbances in Post-Traumatic Stress Disorder: A Review of the Literature". Curr Psychiatry Rep. 17 (6): 41. doi:10.1007/s11920-015-0587-8. PMID 25894359. S2CID 45471039.
- ^ a b c d e Pyke RE (April 2020). "Trazodone in Sexual Medicine: Underused and Overdosed?". Sex Med Rev. 8 (2): 206–16. doi:10.1016/j.sxmr.2018.08.003. PMID 30342856. S2CID 53042045.
- ^ "Riassunto delle caratteristiche del prodotto" [Summary of Product Characteristics] (in Italian). Archived from the original on 25 November 2023.
- ^ "Oleptro (trazodone hydrochloride) extended-release tablets". Pharmacy and Therapeutics. 36 (2): 2–18. 2011. ISSN 1052-1372. PMC 3059557. PMID 21431085.
- ^ "Trazodone (Oral Route) Proper Use Clinic". www.mayoclinic.org – Mayo Clinic. Retrieved 11 February 2020.
- ^ Malhi GS (November 2015). "Antidepressants in bipolar depression: yes, no, maybe?". Evid Based Ment Health. 18 (4): 100–02. doi:10.1136/eb-2015-102229. PMC 11234584. PMID 26459471.
- ^ "Webmd.com". Webmd.com. Retrieved 14 March 2014.
- ^ Warner CH, Bobo W, Warner C, Reid S, Rachal J (August 2006). "Antidepressant discontinuation syndrome". Am Fam Physician. 74 (3): 449–56. PMID 16913164.
- ^ "FDA – Trazodone Prescribing Information" (PDF).
- ^ Wesnes KA, Simpson PM, Christmas L, Anand R, McClelland GR (1989). "The effects of moclobemide on cognition". J. Neural Transm. Suppl. 28: 91–102. PMID 2677245.
- ^ Brunnauer A, Laux G (September 2017). "Driving Under the Influence of Antidepressants: A Systematic Review and Update of the Evidence of Experimental and Controlled Clinical Studies". Pharmacopsychiatry. 50 (5): 173–81. doi:10.1055/s-0043-113572. PMID 28718182. S2CID 3425898.
- ^ a b Schatzberg AF, Nemeroff CB, eds. (2009). Textbook of Psychopharmacology (4th ed.). Washington, D.C.: American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- ^ "Trazodone Product Monograph" (PDF). 2015.
- ^ "Highlights of Prescribing Information" (PDF). U.S. Food and Drug Administration. 2017.
- ^ Abber JC, Lue TF, Luo JA, Juenemann KP, Tanagho EA (May 1987). "Priapism induced by chlorpromazine and trazodone: mechanism of action". J. Urol. 137 (5): 1039–42. doi:10.1016/s0022-5347(17)44355-2. PMID 3573170.
- ^ Chen WH, Chu YH, Chen KY (2018). "Drug-Associated Spontaneous Orgasm: A Case Report and Systematic Review of Literature". Clin Neuropharmacol. 41 (1): 31–37. doi:10.1097/WNF.0000000000000259. PMID 29194112. S2CID 39522632.
- ^ Battaglia C, Venturoli S (October 2009). "Persistent genital arousal disorder and trazodone. Morphometric and vascular modifications of the clitoris. A case report". J Sex Med. 6 (10): 2896–900. doi:10.1111/j.1743-6109.2009.01418.x. PMID 19674253.
- ^ Kalgutkar AS, Henne KR, Lame ME, Vaz AD, Collin C, Soglia JR, et al. (June 2005). "Metabolic activation of the nontricyclic antidepressant trazodone to electrophilic quinone-imine and epoxide intermediates in human liver microsomes and recombinant P4503A4". Chem. Biol. Interact. 155 (1–2): 10–20. Bibcode:2005CBI...155...10K. doi:10.1016/j.cbi.2005.03.036. PMID 15978881.
- ^ a b Otani K, Yasui N, Kaneko S, Ishida M, Ohkubo T, Osanai T, et al. (June 1995). "Trazodone treatment increases plasma prolactin concentrations in depressed patients". Int Clin Psychopharmacol. 10 (2): 115–17. doi:10.1097/00004850-199506000-00009. PMID 7673654. S2CID 41490922.
- ^ Gonçalo AM, Vieira-Coelho MA (November 2021). "The effects of trazodone on human cognition: a systematic review". Eur J Clin Pharmacol. 77 (11): 1623–37. doi:10.1007/s00228-021-03161-6. PMC 8182348. PMID 34097124.
- ^ Kolla BP, Mansukhani MP, Bostwick JM (April 2018). "The influence of antidepressants on restless legs syndrome and periodic limb movements: A systematic review". Sleep Med Rev. 38: 131–40. doi:10.1016/j.smrv.2017.06.002. PMID 28822709.
- ^ Oderda LH, Young JR, Asche CV, Pepper GA (2012). "Psychotropic-related hip fractures: meta-analysis of first-generation and second-generation antidepressant and antipsychotic drugs". Ann Pharmacother. 46 (7–8): 917–28. doi:10.1345/aph.1Q589. PMID 22811347. S2CID 22681213.
- ^ Einarson A, Bonari L, Voyer-Lavigne S, Addis A, Matsui D, Johnson Y, et al. (March 2003). "A multicentre prospective controlled study to determine the safety of trazodone and nefazodone use during pregnancy". Can J Psychiatry. 48 (2): 106–10. doi:10.1177/070674370304800207. PMID 12655908.
- ^ Verbeeck RK, Ross SG, McKenna EA (September 1986). "Excretion of trazodone in breast milk". Br J Clin Pharmacol. 22 (3): 367–70. doi:10.1111/j.1365-2125.1986.tb02903.x. PMC 1401139. PMID 3768252.
- ^ a b Cushing TA (24 April 2018). "Selective Serotonin Reuptake Inhibitor Toxicity". Medscape. WebMD LLC. Retrieved 22 December 2018.
- ^ Martínez MA, Ballesteros S, Sánchez de la Torre C, Almarza E (2005). "Investigation of a fatality due to trazodone poisoning: case report and literature review". J Anal Toxicol. 29 (4): 262–28. doi:10.1093/jat/29.4.262. PMID 15975258.
- ^ de Meester A, Carbutti G, Gabriel L, Jacques JM (2001). "Fatal overdose with trazodone: case report and literature review". Acta Clin Belg. 56 (4): 258–61. doi:10.1179/acb.2001.038. PMID 11603256. S2CID 21487479.
- ^ Rakel RE (1987). "The greater safety of trazodone over tricyclic antidepressant agents: 5-year experience in the United States". Psychopathology. 20 (Suppl 1): 57–63. doi:10.1159/000284524. PMID 3321131.
- ^ a b Rotzinger S, Fang J, Baker GB (June 1998). "Trazodone is metabolized to m-chlorophenylpiperazine by CYP3A4 from human sources". Drug Metabolism and Disposition. 26 (6): 572–75. PMID 9616194.
- ^ a b c "Table of Substrates, Inhibitors and Inducers". U.S. Food and Drug Administration. 5 June 2023. Retrieved 5 July 2024.
- ^ Yasui N, Otani K, Kaneko S, Ohkubo T, Osanai T, Ishida M, et al. (August 1995). "Inhibition of trazodone metabolism by thioridazine in humans". Ther Drug Monit. 17 (4): 333–35. doi:10.1097/00007691-199508000-00003. PMID 7482685. S2CID 1979283.
- ^ Sager JE, Lutz JD, Foti RS, Davis C, Kunze KL, Isoherranen N (June 2014). "Fluoxetine- and norfluoxetine-mediated complex drug-drug interactions: in vitro to in vivo correlation of effects on CYP2D6, CYP2C19, and CYP3A4". Clin Pharmacol Ther. 95 (6): 653–62. doi:10.1038/clpt.2014.50. PMC 4029899. PMID 24569517.
- ^ a b c d Maes M, Westenberg H, Vandoolaeghe E, Demedts P, Wauters A, Neels H, et al. (October 1997). "Effects of trazodone and fluoxetine in the treatment of major depression: therapeutic pharmacokinetic and pharmacodynamic interactions through formation of meta-chlorophenylpiperazine". Journal of Clinical Psychopharmacology. 17 (5): 358–64. doi:10.1097/00004714-199710000-00004. PMID 9315986.
- ^ a b Mihara K, Otani K, Suzuki A, Yasui N, Nakano H, Meng X, et al. (September 1997). "Relationship between the CYP2D6 genotype and the steady-state plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine". Psychopharmacology (Berl). 133 (1): 95–98. doi:10.1007/s002130050376. PMID 9335086. S2CID 11521715.
- ^ a b Saiz-Rodríguez M, Belmonte C, Derqui-Fernández N, Cabaleiro T, Román M, Ochoa D, et al. (November 2017). "Pharmacogenetics of trazodone in healthy volunteers: association with pharmacokinetics, pharmacodynamics and safety". Pharmacogenomics. 18 (16): 1491–502. doi:10.2217/pgs-2017-0116. PMID 29061081.
- ^ a b Ishida M, Otani K, Kaneko S, Ohkubo T, Osanai T, Yasui N, et al. (September 1995). "Effects of various factors on steady state plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine". Int Clin Psychopharmacol. 10 (3): 143–46. doi:10.1097/00004850-199510030-00002. PMID 8675966.
- ^ a b c d e f Halman A, Kong G, Sarris J, Perkins D (January 2024). "Drug-drug interactions involving classic psychedelics: A systematic review". J Psychopharmacol. 38 (1): 3–18. doi:10.1177/02698811231211219. PMC 10851641. PMID 37982394.
- ^ a b c d e f Settimo L, Taylor D (January 2018). "Evaluating the dose-dependent mechanism of action of trazodone by estimation of occupancies for different brain neurotransmitter targets". J Psychopharmacol. 32 (1): 96–104. doi:10.1177/0269881117742101. PMID 29332554. S2CID 28804036.
- ^ a b Bonson KR, Buckholtz JW, Murphy DL (June 1996). "Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans". Neuropsychopharmacology. 14 (6): 425–436. doi:10.1016/0893-133X(95)00145-4. PMID 8726753.
- ^ a b c Yates G, Melon E (January 2024). "Trip-killers: a concerning practice associated with psychedelic drug use". Emerg Med J. 41 (2): 112–113. doi:10.1136/emermed-2023-213377. PMID 38123961.
Table 1 Trip-killers recommended by Reddit users [...] Antidepressants, n=100 [...] Drug Name: Trazodone; N: 77. [...] Table 2 Trip-killer doses recommended by Reddit users: Drug name: Trazodone. Dose range: 50–150 mg.
- ^ a b c Suran M (February 2024). "Study Finds Hundreds of Reddit Posts on "Trip-Killers" for Psychedelic Drugs". JAMA. 331 (8): 632–634. doi:10.1001/jama.2023.28257. PMID 38294772.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 25 May 2018.
- ^ a b c d Owens MJ, Morgan WN, Plott SJ, Nemeroff CB (1997). "Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites". J. Pharmacol. Exp. Ther. 283 (3): 1305–22. PMID 9400006.
- ^ a b c Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- ^ a b c d e f g Cusack B, Nelson A, Richelson E (1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology. 114 (4): 559–65. doi:10.1007/bf02244985. PMID 7855217. S2CID 21236268.
- ^ a b c d e f g h i j Hamik A, Peroutka SJ (1989). "1-(m-chlorophenyl)piperazine (mCPP) interactions with neurotransmitter receptors in the human brain". Biol. Psychiatry. 25 (5): 569–75. doi:10.1016/0006-3223(89)90217-5. PMID 2537663. S2CID 46730665.
- ^ Boess FG, Martin IL (1994). "Molecular biology of 5-HT receptors". Neuropharmacology. 33 (3–4): 275–317. doi:10.1016/0028-3908(94)90059-0. PMID 7984267. S2CID 35553281.
- ^ Hamblin MW, Metcalf MA (1991). "Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor". Mol. Pharmacol. 40 (2): 143–48. PMID 1652050.
- ^ a b c Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, et al. (2004). "Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors". Naunyn-Schmiedeberg's Arch. Pharmacol. 370 (2): 114–23. doi:10.1007/s00210-004-0951-4. PMID 15322733. S2CID 8938111.
- ^ Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, et al. (1995). "The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors". Br. J. Pharmacol. 115 (4): 622–28. doi:10.1111/j.1476-5381.1995.tb14977.x. PMC 1908489. PMID 7582481.
- ^ a b Rothman RB, Baumann MH (2009). "Serotonergic drugs and valvular heart disease". Expert Opin Drug Saf. 8 (3): 317–29. doi:10.1517/14740330902931524. PMC 2695569. PMID 19505264.
- ^ Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. (2000). "Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications". Circulation. 102 (23): 2836–41. doi:10.1161/01.cir.102.23.2836. PMID 11104741.
- ^ Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, et al. (1999). "Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells". Br. J. Pharmacol. 128 (1): 13–20. doi:10.1038/sj.bjp.0702751. PMC 1571597. PMID 10498829.
- ^ Bentley JM, Adams DR, Bebbington D, Benwell KR, Bickerdike MJ, Davidson JE, et al. (2004). "Indoline derivatives as 5-HT(2C) receptor agonists". Bioorg. Med. Chem. Lett. 14 (9): 2367–70. doi:10.1016/j.bmcl.2003.05.001. PMID 15081042.
- ^ Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, et al. (1997). "RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist". Neuropharmacology. 36 (4–5): 621–29. doi:10.1016/s0028-3908(97)00049-x. PMID 9225287. S2CID 24930608.
- ^ a b c d Richelson E, Nelson A (1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. PMID 6086881.
- ^ a b Stanton T, Bolden-Watson C, Cusack B, Richelson E (1993). "Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics". Biochem. Pharmacol. 45 (11): 2352–54. doi:10.1016/0006-2952(93)90211-e. PMID 8100134.
- ^ a b Raffa RB, Shank RP, Vaught JL (1992). "Etoperidone, trazodone and MCPP: in vitro and in vivo identification of serotonin 5-HT1A (antagonistic) activity". Psychopharmacology. 108 (3): 320–26. doi:10.1007/BF02245118. PMID 1387963. S2CID 24965789.
- ^ a b Odagaki Y, Toyoshima R, Yamauchi T (May 2005). "Trazodone and its active metabolite m-chlorophenylpiperazine as partial agonists at 5-HT1A receptors assessed by [35S]GTPgammaS binding". J. Psychopharmacol. (Oxford). 19 (3): 235–41. doi:10.1177/0269881105051526. PMID 15888508. S2CID 27389008.
- ^ Krystal AD, Richelson E, Roth T (2013). "Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications". Sleep Med Rev. 17 (4): 263–72. doi:10.1016/j.smrv.2012.08.001. PMID 23357028.
- ^ Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 586–. ISBN 978-0-7817-6879-5.
- ^ Kahn RS, Wetzler S (1991). "m-Chlorophenylpiperazine as a probe of serotonin function". Biol. Psychiatry. 30 (11): 1139–66. doi:10.1016/0006-3223(91)90184-n. PMID 1663792. S2CID 13007057.
- ^ a b Melzacka M, Rurak A, Vetulani J (1980). "Preliminary study of the biotransformation of two new drugs, trazodone and etoperidone". Polish Journal of Pharmacology and Pharmacy. 32 (4): 551–56. PMID 7255270.
- ^ a b Fong MH, Garattini S, Caccia S (October 1982). "1-m-Chlorophenylpiperazine is an active metabolite common to the psychotropic drugs trazodone, etoperidone and mepiprazole". The Journal of Pharmacy and Pharmacology. 34 (10): 674–75. doi:10.1111/j.2042-7158.1982.tb04701.x. PMID 6128394. S2CID 44968564.
- ^ a b c d e f Al-Yassiri MM, Ankier SI, Bridges PK (June 1981). "Trazodone--a new antidepressant". Life Sci. 28 (22): 2449–2458. doi:10.1016/0024-3205(81)90586-5. PMID 7019617.
- ^ a b c d e Ayd FJ, Settle EC (1982). "Trazodone: a novel, broad-spectrum antidepressant". Mod Probl Pharmacopsychiatry. Modern Trends in Pharmacopsychiatry. 18: 49–69. doi:10.1159/000406236. ISBN 978-3-8055-3428-4. PMID 6124884.
- ^ a b c Rawls WN (January 1982). "Trazodone (Desyrel, Mead-Johnson Pharmaceutical Division)". Drug Intell Clin Pharm. 16 (1): 7–13. doi:10.1177/106002808201600102. PMID 7032872.
- ^ Tucker JC, File SE (1986). "The effects of tricyclic and 'atypical' antidepressants on spontaneous locomotor activity in rodents". Neurosci Biobehav Rev. 10 (2): 115–121. doi:10.1016/0149-7634(86)90022-9. PMID 3737024.
- ^ Baran L, Maj J, Rogóz Z, Skuza G (1979). "On the central antiserotonin action of trazodone". Pol J Pharmacol Pharm. 31 (1): 25–33. PMID 482164.
- ^ a b Balsara JJ, Jadhav SA, Gaonkar RK, Gaikwad RV, Jadhav JH (May 2005). "Effects of the antidepressant trazodone, a 5-HT 2A/2C receptor antagonist, on dopamine-dependent behaviors in rats". Psychopharmacology (Berl). 179 (3): 597–605. doi:10.1007/s00213-004-2095-0. PMID 15614572.
- ^ a b c d e Sarwar AI (2018). "Trazodone and Parkinsonism: The Link Strengthens". Clin Neuropharmacol. 41 (3): 106–108. doi:10.1097/WNF.0000000000000278. PMID 29634584.
Although the exact underlying mechanism causing parkinsonism after the exposure of trazodone in this or any of the previously described cases remains elusive, it likely involves trazodone's inhibitory effect, either directly or via the serotonergic system on striatal dopaminergic neurotransmission.7,8,12–16 It is known that serotonin (5-hydroxytryptamine [5-HT]), via stimulation of 5-HT2C receptors, exerts a tonic inhibitory influence on dopaminergic neurotransmission, whereas activation of 5-HT2A receptors enhances dopaminergic neurotransmission. The antidepressant trazodone is a 5-HT2A and 5-HT2C receptor antagonist.7,16 However, its antagonism at 5-HT2A receptor is more robust than that at 5-HT2C receptors (15-fold difference).7,8 Hence, the differential antagonist effect of trazodone with respect to 5-HT2A versus 5-HT2C receptors "at a certain dose point" could potentially result in inhibition of dopaminergic neurotransmission in the striatonigral region. In addition, a direct dose-dependent blocking effect of trazodone on postsynaptic striatal D2 dopamine receptors has been demonstrated in the rat model.8
- ^ Höllerhage M (2019). "Secondary parkinsonism due to drugs, vascular lesions, tumors, trauma, and other insults". Int Rev Neurobiol. International Review of Neurobiology. 149: 377–418. doi:10.1016/bs.irn.2019.10.010. ISBN 978-0-12-817730-3. PMID 31779822.
- ^ a b c Marek GJ, McDougle CJ, Price LH, Seiden LS (1992). "A comparison of trazodone and fluoxetine: implications for a serotonergic mechanism of antidepressant action". Psychopharmacology. 109 (1–2): 2–11. doi:10.1007/BF02245475. PMID 1365657. S2CID 25140746.
- ^ Vanina Y, Podolskaya A, Sedky K, Shahab H, Siddiqui A, Munshi F, et al. (July 2002). "Body weight changes associated with psychopharmacology". Psychiatr Serv. 53 (7): 842–47. doi:10.1176/appi.ps.53.7.842. PMID 12096167.
- ^ Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, McGuire H, et al. (January 2010). "Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression: systematic review and meta-analysis". CNS Drugs. 24 (1): 35–53. doi:10.2165/11319480-000000000-00000. PMID 20030418. S2CID 7459081.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Kinney GG, Griffith JC, Hudzik TJ (July 1998). "Antidepressant-like effects of 5-hydroxytryptamine1A receptor agonists on operant responding under a response duration differentiation schedule". Behav Pharmacol. 9 (4): 309–18. doi:10.1097/00008877-199807000-00002. PMID 10065919.
- ^ a b c d e f Stahl SM (2013). Stahl's Essential Psychopharmacology (4th ed.). Cambridge University Press. ISBN 978-1-107-68646-5.
- ^ Asayesh K (December 1986). "Combination of trazodone and phenothiazines: a possible additive hypotensive effect". Canadian Journal of Psychiatry. 31 (9): 857–58. doi:10.1177/070674378603100913. PMID 3802006. S2CID 43202340.
- ^ Mihara K, Yasui-Furukori N, Kondo T, Ishida M, Ono S, Ohkubo T, et al. (August 2002). "Relationship between plasma concentrations of trazodone and its active metabolite, m-chlorophenylpiperazine, and its clinical effect in depressed patients". Therapeutic Drug Monitoring. 24 (4): 563–66. doi:10.1097/00007691-200208000-00016. PMID 12142643. S2CID 25709000.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Li AA, Marek GJ, Hand TH, Seiden LS (February 1990). "Antidepressant-like effects of trazodone on a behavioral screen are mediated by trazodone, not the metabolite m-chlorophenylpiperazine". European Journal of Pharmacology. 177 (3): 137–44. doi:10.1016/0014-2999(90)90263-6. PMID 2311675.
- ^ Vetulani J, Sansone M, Baran L, Hano J (1984). "Opposite action of m-chlorophenylpiperazine on avoidance depression induced by trazodone and pimozide in CD-1 mice". Psychopharmacology. 83 (2): 166–68. doi:10.1007/BF00429728. PMID 6431467. S2CID 38913696.
- ^ Kast RE (2009). "Trazodone generates m-CPP: in 2008 risks from m-CPP might outweigh benefits of trazodone". The World Journal of Biological Psychiatry. 10 (4 Pt 2): 682–85. doi:10.1080/15622970902836022. PMID 19384678. S2CID 901077.
- ^ Workman EA, Tellian F, Short D (May 1992). "Trazodone induction of migraine headache through mCPP". The American Journal of Psychiatry. 149 (5): 712b–712. doi:10.1176/ajp.149.5.712b. PMID 1575270.
- ^ a b "Trazodone". www.drugbank.ca. Retrieved 31 January 2019.
- ^ Mihara K, Kondo T, Suzuki A, Yasui-Furukori N, Ono S, Otani K, et al. (May 2001). "Effects of genetic polymorphism of CYP1A2 inducibility on the steady-state plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine in depressed Japanese patients". Pharmacol Toxicol. 88 (5): 267–70. doi:10.1034/j.1600-0773.2001.d01-115.x (inactive 12 November 2024). PMID 11393588.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Garattini S (1985). "Active drug metabolites. An overview of their relevance in clinical pharmacokinetics". Clinical Pharmacokinetics. 10 (3): 216–27. doi:10.2165/00003088-198510030-00002. PMID 2861928. S2CID 21305772.
- ^ Vaz RJ, Klabunde T (2008). Antitargets: Prediction and Prevention of Drug Side Effects. John Wiley & Sons. pp. 149–. ISBN 978-3-527-62147-7.
- ^ Logan BK, Costantino AG, Rieders EF, Sanders D (November 2010). "Trazodone, meta-chlorophenylpiperazine (an hallucinogenic drug and trazodone metabolite), and the hallucinogen trifluoromethylphenylpiperazine cross-react with the EMIT®II ecstasy immunoassay in urine". J Anal Toxicol. 34 (9): 587–89. doi:10.1093/jat/34.9.587. PMID 21073812.
- ^ Jauch R, Kopitar Z, Prox A, Zimmer A (1976). "[Pharmacokinetics and metabolism of trazodone in man (author's transl)]". Arzneimittel-Forschung (in German). 26 (11): 2084–89. PMID 1037253.
- ^ Akritopoulou-Zanze I (2012). "6. Arylpiperazine-Based 5-HT1A Receptor Partial Agonists and 5-HT2A Antagonists for the Treatment of Autism, Depression, Anxiety, Psychosis, and Schizophrenia". In Dinges J, Lamberth C (eds.). Bioactive heterocyclic compound classes pharmaceuticals. Weinheim: Wiley-VCH. ISBN 978-3-527-66445-0.
- ^ Dörwald FZ, ed. (2012). "46. Arylalkylamines". Lead optimization for medicinal chemists : pharmacokinetic properties of functional groups and organic compounds. Weinheim: Wiley-VCH. ISBN 978-3-527-64564-0.
- ^ Gorecki DK, Verbeeck RK (1987). "Trazondone Hydrochloride". In Forey K (ed.). Profiles of Drug Substances, Excipients and Related Methodology Vol. 16. Academic Press. p. 695. ISBN 978-0-08-086111-1.
- ^ Wegener G (30 March 2016). "Ban & Silvestrini's Trazodone". International Network for the History of Neuropsychopharmacology. Archived from the original on 20 March 2017. Retrieved 4 June 2017.
- ^ Silvestrini B (1989). "Trazodone: from the mental pain to the "dys-stress" hypothesis of depression". Clinical Neuropharmacology. 12 (Suppl 1): S4–10. doi:10.1097/00002826-198901001-00002. PMID 2568177. S2CID 34798378.
- ^ "Trazodone: Common sleep drug is little-known antidepressant". Consumer Reports. August 2015.
- ^ Eisen MS, Taylor DB, Riblet LA (2012). "Atypical Psychotropic Agents". In Williams M, Malick JB (eds.). Drug Discovery and Development. Springer Science & Business Media. p. 388. ISBN 978-1-4612-4828-6.
- ^ Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. ISBN 978-1-4757-2085-3.
- ^ a b c Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1050–52. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 279–. ISBN 978-94-011-4439-1.
- ^ a b c "Trazodone". Drugs.com.
- ^ Ramli FF, Azizi MH, Syed Hashim SA (2021). "Treatments of Sexual Dysfunction in Opioid Substitution Therapy Patients: A Systematic Review and Meta-Analysis". Int J Med Sci. 18 (11): 2372–80. doi:10.7150/ijms.57641. PMC 8100642. PMID 33967614.
- ^ Fink HA, MacDonald R, Rutks IR, Wilt TJ (September 2003). "Trazodone for erectile dysfunction: a systematic review and meta-analysis". BJU Int. 92 (4): 441–46. doi:10.1046/j.1464-410X.2003.04358.x. PMID 12930437. S2CID 7499389.
- ^ a b "Bupropion/trazodone - AdisInsight". adisinsight.springer.com.
- ^ Miller MK, Smith JR, Norman JJ, Clayton AH (September 2018). "Expert opinion on existing and developing drugs to treat female sexual dysfunction". Expert Opin Emerg Drugs. 23 (3): 223–30. doi:10.1080/14728214.2018.1527901. PMID 30251897. S2CID 52813769.
- ^ a b Belkin ZR, Krapf JM, Goldstein AT (February 2015). "Drugs in early clinical development for the treatment of female sexual dysfunction". Expert Opin Investig Drugs. 24 (2): 159–67. doi:10.1517/13543784.2015.978283. PMID 25376023. S2CID 207477620.
- ^ Kolla BP, Mansukhani MP, Schneekloth T (2011). "Pharmacological treatment of insomnia in alcohol recovery: a systematic review". Alcohol Alcohol. 46 (5): 578–85. doi:10.1093/alcalc/agr073. PMID 21715413.
- ^ AbdelFattah MR, Jung SW, Greenspan MA, Padilla M, Enciso R (June 2020). "Efficacy of Antidepressants in the Treatment of Obstructive Sleep Apnea Compared to Placebo. A Systematic Review with Meta-Analyses". Sleep Breath. 24 (2): 443–53. doi:10.1007/s11325-019-01954-9. PMID 31720982. S2CID 207939482.
- ^ Smales ET, Edwards BA, Deyoung PN, McSharry DG, Wellman A, Velasquez A, et al. (May 2015). "Trazodone Effects on Obstructive Sleep Apnea and Non-REM Arousal Threshold". Ann Am Thorac Soc. 12 (5): 758–64. doi:10.1513/AnnalsATS.201408-399OC. PMC 4418332. PMID 25719754.
- ^ Eckert DJ, Malhotra A, Wellman A, White DP (April 2014). "Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold". Sleep. 37 (4): 811–19. doi:10.5665/sleep.3596. PMC 4044741. PMID 24899767.
- ^ Martinon-Torres G, Fioravanti M, Grimley EJ (October 2004). "Trazodone for agitation in dementia". Cochrane Database Syst Rev (4): CD004990. doi:10.1002/14651858.CD004990. PMID 15495135.
- ^ Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P (February 2011). "Antidepressants for agitation and psychosis in dementia". Cochrane Database Syst Rev (2): CD008191. doi:10.1002/14651858.CD008191.pub2. PMID 21328305.
- ^ McCleery J, Sharpley AL (November 2020). "Pharmacotherapies for sleep disturbances in dementia". Cochrane Database Syst Rev. 2020 (11): CD009178. doi:10.1002/14651858.CD009178.pub4. PMC 8094738. PMID 33189083.
- ^ Nardell M, Tampi RR (March 2014). "Pharmacological treatments for frontotemporal dementias: a systematic review of randomized controlled trials". Am J Alzheimers Dis Other Demen. 29 (2): 123–32. doi:10.1177/1533317513507375. PMC 10852735. PMID 24164931. S2CID 13491951.
- ^ Chen A, Copeli F, Metzger E, Cloutier A, Osser DN (January 2021). "The Psychopharmacology Algorithm Project at the Harvard South Shore Program: An update on management of behavioral and psychological symptoms in dementia". Psychiatry Res. 295: 113641. doi:10.1016/j.psychres.2020.113641. PMID 33340800. S2CID 228158773.
- ^ Trieu C, Gossink F, Stek ML, Scheltens P, Pijnenburg YA, Dols A (March 2020). "Effectiveness of Pharmacological Interventions for Symptoms of Behavioral Variant Frontotemporal Dementia: A Systematic Review". Cogn Behav Neurol. 33 (1): 1–15. doi:10.1097/WNN.0000000000000217. PMID 32132398. S2CID 212417082.
- ^ Chow TW (October 2005). "Treatment approaches to symptoms associated with frontotemporal degeneration". Curr Psychiatry Rep. 7 (5): 376–80. doi:10.1007/s11920-005-0040-5. PMID 16216158. S2CID 8766330.
- ^ a b c Singh SP, Singh V, Kar N, Chan K (September 2010). "Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis". Br J Psychiatry. 197 (3): 174–79. doi:10.1192/bjp.bp.109.067710. PMID 20807960.
- ^ Terevnikov V, Joffe G, Stenberg JH (May 2015). "Randomized Controlled Trials of Add-On Antidepressants in Schizophrenia". Int J Neuropsychopharmacol. 18 (9): pyv049. doi:10.1093/ijnp/pyv049. PMC 4576515. PMID 25991654.
- ^ Bacaltchuk J, Hay P (2003). "Antidepressants versus placebo for people with bulimia nervosa". Cochrane Database Syst Rev (4): CD003391. doi:10.1002/14651858.CD003391. PMC 6991155. PMID 14583971.
- ^ Constantin D, Dinu EA, Rogozea L, Burtea V, Leasu FG (2020). "Therapeutic Interventions for Adjustment Disorder: A Systematic Review". Am J Ther. 27 (4): e375–86. doi:10.1097/MJT.0000000000001170. PMID 32520732. S2CID 219586994.
- ^ Chisini LA, San Martin AS, Cademartori MG, Boscato N, Correa MB, Goettems ML (February 2020). "Interventions to reduce bruxism in children and adolescents: a systematic scoping review and critical reflection". Eur J Pediatr. 179 (2): 177–89. doi:10.1007/s00431-019-03549-8. PMID 31858254. S2CID 209411316.
- ^ Victor S, Ryan SW (2003). Hobson A (ed.). "Drugs for preventing migraine headaches in children". Cochrane Database Syst Rev (4): CD002761. doi:10.1002/14651858.CD002761. PMID 14583952.
- ^ Damen L, Bruijn J, Verhagen AP, Berger MY, Passchier J, Koes BW (May 2006). "Prophylactic treatment of migraine in children. Part 2. A systematic review of pharmacological trials". Cephalalgia. 26 (5): 497–505. doi:10.1111/j.1468-2982.2005.01047.x. PMID 16674757. S2CID 37252070.
- ^ El-Chammas K, Keyes J, Thompson N, Vijayakumar J, Becher D, Jackson JL (March 2013). "Pharmacologic treatment of pediatric headaches: a meta-analysis". JAMA Pediatr. 167 (3): 250–28. doi:10.1001/jamapediatrics.2013.508. PMC 4692044. PMID 23358935.
- ^ Shamliyan TA, Kane RL, Ramakrishnan R, Taylor FR (October 2013). "Episodic migraines in children: limited evidence on preventive pharmacological treatments". J Child Neurol. 28 (10): 1320–41. doi:10.1177/0883073813488659. PMID 23752070. S2CID 9178257.
- ^ Migliorini F, Maffulli N, Eschweiler J, Knobe M, Tingart M, Colarossi G (February 2022). "Pharmacological management of fibromyalgia: a Bayesian network meta-analysis". Expert Rev Clin Pharmacol. 15 (2): 205–14. doi:10.1080/17512433.2022.2044792. PMID 35184627. S2CID 247006915.
- ^ Farag AM, Kuten-Shorrer M, Natto Z, Ariyawardana A, Mejia LM, Albuquerque R, et al. (March 2021). "WWOM VII: Effectiveness of systemic pharmacotherapeutic interventions in the management of BMS: A systematic review and meta-analysis". Oral Dis. 29 (2): 343–368. doi:10.1111/odi.13817. PMID 33713052. S2CID 232217908.
- ^ Liu YF, Kim Y, Yoo T, Han P, Inman JC (April 2018). "Burning mouth syndrome: a systematic review of treatments". Oral Dis. 24 (3): 325–34. doi:10.1111/odi.12660. PMID 28247977.
- ^ Koman LA, Smith BP, Ekman EF, Smith TL (2005). "Complex regional pain syndrome". Instr Course Lect. 54: 11–20. PMID 15948431.
- ^ Xiong N, Duan Y, Wei J, Mewes R, Leonhart R (2018). "Antidepressants vs. Placebo for the Treatment of Functional Gastrointestinal Disorders in Adults: A Systematic Review and Meta-Analysis". Front Psychiatry. 9: 659. doi:10.3389/fpsyt.2018.00659. PMC 6288425. PMID 30564156.
- ^ Nguyen TM, Eslick GD (March 2012). "Systematic review: the treatment of noncardiac chest pain with antidepressants". Aliment Pharmacol Ther. 35 (5): 493–500. doi:10.1111/j.1365-2036.2011.04978.x. PMID 22239853. S2CID 21793648.
- ^ Hershcovici T, Achem SR, Jha LK, Fass R (January 2012). "Systematic review: the treatment of noncardiac chest pain". Aliment Pharmacol Ther. 35 (1): 5–14. doi:10.1111/j.1365-2036.2011.04904.x. PMID 22077344. S2CID 293910.
- ^ Berends HI, Nijlant JM, Movig KL, Van Putten MJ, Jannink MJ, Ijzerman MJ (December 2009). "The clinical use of drugs influencing neurotransmitters in the brain to promote motor recovery after stroke; a Cochrane systematic review". Eur J Phys Rehabil Med. 45 (4): 621–30. PMID 20032921.
- ^ Erickson A, Harbin K, MacPherson J, Rundle K, Overall KL (September 2021). "A review of pre-appointment medications to reduce fear and anxiety in dogs and cats at veterinary visits". Can Vet J. 62 (9): 952–60. PMC 8360309. PMID 34475580.
- ^ Lefman SH, Prittie JE (March 2019). "Psychogenic stress in hospitalized veterinary patients: Causation, implications, and therapies". J Vet Emerg Crit Care (San Antonio). 29 (2): 107–20. doi:10.1111/vec.12821. PMID 30861632. S2CID 76667125.
- ^ Sinn L (May 2018). "Advances in Behavioral Psychopharmacology". Vet Clin North Am Small Anim Pract. 48 (3): 457–71. doi:10.1016/j.cvsm.2017.12.011. PMID 29415813.
