From Wikipedia, the free encyclopedia
First generation antihistamine
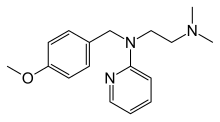
Mepyramine , also known as pyrilamine , is a first-generation antihistamine , targeting the H1 receptor as an inverse agonist .[ 1] drowsiness .[ 2] maleate salt, pyrilamine maleate .
The medication has negligible anticholinergic activity, with 130,000-fold selectivity for the histamine H1 receptor over the muscarinic acetylcholine receptors (for comparison, diphenhydramine had 20-fold selectivity for the H1 receptor).[ 3]
It was patented in 1943 and came into medical use in 1949.[ 4] [ 5] sleep aids such as Alva-Tranquil, Dormin, Sedacaps, Sominex , Nytol, and many others.[ 5] Food and Drug Administration (FDA) included it in the list of chemicals and compounds barred from use in over-the-counter (OTC) nighttime sleep aid products in 1989.[ 6]
It is used in over-the-counter combination products to treat the common cold and menstrual symptoms such as Midol Complete .[ 7] [ 8] [ 1]
Psychedelics (5-HT2A
Benzofurans Lyserg‐ Phenethyl‐
2C-x
3C-x 4C-x DOx HOT-x MDxx Mescaline (subst.) TMAs
TMA
TMA-2
TMA-3
TMA-4
TMA-5
TMA-6 Others
Piperazines Tryptamines
alpha -alkyltryptaminesx -DALT x -DET x -DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4,N,N-TMT 4-Propionyloxy-DMT 5,6-diBr-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT 5-MeO-α,N,N-TMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT Ibogaine-related x -MET x -MiPT Others
Others
Dissociatives (NMDAR antagonists )
Deliriants (mAChR antagonists ) Others
H1
Agonists Antagonists
Others: Atypical antipsychotics (e.g., aripiprazole , asenapine , brexpiprazole , brilaroxazine , clozapine , iloperidone , olanzapine , paliperidone , quetiapine , risperidone , ziprasidone , zotepine )Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Tetracyclic antidepressants (e.g., amoxapine , loxapine , maprotiline , mianserin , mirtazapine , oxaprotiline )Tricyclic antidepressants (e.g., amitriptyline , butriptyline , clomipramine , desipramine , dosulepin (dothiepin) , doxepin , imipramine , iprindole , lofepramine , nortriptyline , protriptyline , trimipramine )Typical antipsychotics (e.g., chlorpromazine , flupenthixol , fluphenazine , loxapine , perphenazine , prochlorperazine , thioridazine , thiothixene )
H2
H3
H4
DAT Tooltip Dopamine transporter (DRIs Tooltip Dopamine reuptake inhibitors )
NET Tooltip Norepinephrine transporter (NRIs Tooltip Norepinephrine reuptake inhibitors )
Others: Antihistamines (e.g., brompheniramine , chlorphenamine , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., ketamine , phencyclidine )Dopexamine Ephenidine Ginkgo biloba Indeloxazine Nefazodone Opioids (e.g., desmetramadol , methadone , pethidine (meperidine) , tapentadol , tramadol , levorphanol )
SERT Tooltip Serotonin transporter (SRIs Tooltip Serotonin reuptake inhibitors )
Others: A-80426 Amoxapine Antihistamines (e.g., brompheniramine , chlorphenamine , dimenhydrinate , diphenhydramine , mepyramine (pyrilamine) , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., 3-MeO-PCP , esketamine , ketamine , methoxetamine , phencyclidine )Cyclobenzaprine Delucemine Dextromethorphan Dextrorphan Efavirenz Hypidone Medifoxamine Mesembrine Mifepristone MIN-117 (WF-516) N-Me-5-HT Opioids (e.g., dextropropoxyphene , methadone , pethidine (meperidine) , levorphanol , tapentadol , tramadol )Roxindole
VMATs Tooltip Vesicular monoamine transporters Others