List of AM cannabinoids
Appearance
(Redirected from AM-926)
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are:
| Name | Class | Ki / nM at CB1 | Ki / nM at CB2 | Selectivity | CLogP | Structure | Description |
|---|---|---|---|---|---|---|---|
| AM-087 | Dibenzopyran | 0.43 | 6.47 | 
|
An analgesic CB1 agonist derived from Δ8-THC substituted with a side chain on the 3-position, roughly 100 times as potent as THC. | ||
| AM-251 | Pyrazole derivative | 7.5 | 7.08 | 
|
An inverse agonist at the CB1 cannabinoid receptor that is structurally related to SR141716A (rimonabant), but has a higher binding affinity.[1] | ||
| AM-279 | A Schedule I substance in Alabama.[2] | ||||||
| AM-281 | N-(morpholin-4-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-pyrazole-3-carboxamide[1] | ||||||
| AM-356 | 17.9 | 868 | 5.55 | 
|
A synthetically created stable chiral analog of anandamide, it acts on both cannabinoid receptors.[3] | ||
| AM-374 | Palmitylsulfonyl fluoride[4] | ||||||
| AM-381 | Stearylsulfonyl fluoride | ||||||
| AM-404 | 7.02 | 
|
An active metabolite of paracetamol (acetaminophen) and a likely inhibitor of fatty acid amide hydrolase (FAAH) | ||||
| AM-411 | 6.80 | 52.0 | 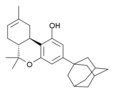
|
An adamantyl-substituted derivative of Δ8-THC, it is a potent and fairly selective CB1 full agonist and a moderately potent CB2 agonist. | |||
| AM-630 | 32.1 | CB2 (165×) | 4.19 | 
|
A potent and selective inverse agonist for the cannabinoid receptor CB2 and a weak partial agonist at CB1. | ||
| AM-661 | 1-(N-methyl-2-piperidine)methyl-2-methyl-3-(2-iodo)benzoylindole[5] | ||||||
| AM-678 | 9.00 ± 5.00 | 2.94 ± 2.65 | CB2 | 5.68 | 
|
Another name for JWH-018, it is a full agonist at both cannabinoid receptors with some selectivity for CB2. | |
| AM-679 | 13.5 | 49.5 | 6.04 | 
|
An iodobenzoylindole which acts as a moderately potent agonist for both cannabinoid receptors. | ||
| AM-694 | 0.08 | 1.44 | CB1 (18×) | 5.54 | 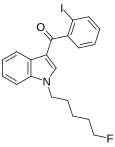
|
An iodobenzoylindole which acts as a potent and selective agonist for the CB1 cannabinoid receptor.[6] | |
| AM-735 | 8.9 | 7.4 | 3-bornyl-Δ8-THC, a mixed CB1 / CB2 agonist.[7] | ||||
| AM-855 | 22.3 | 58.6 | CB1 | 7.1 | 
|
An analgesic derivative of Δ8-tetrahydrocannabinol, it is an agonist at both CB1 and CB2 with moderate selectivity for CB1. | |
| AM-881 | 5.3 | 95 | A chlorine-substituted stereoisomer of anandamide.[3] | ||||
| AM-883 | 9.9 | 226 | An allyl-substituted stereoisomer of anandamide.[3] | ||||
| AM-905 | 1.2 | 5.3 | CB1 | 4.98 | 
|
A potent and reasonably selective agonist for the CB1 cannabinoid receptor. | |
| AM-906 | 0.8 | 9.5 | CB1 | 4.98 | 
|
A potent and dodecally selective agonist for the CB1 cannabinoid receptor. | |
| AM-919 | 2.2 | 3.4 | CB1 | 6.21 | 
|
A potent agonist at both CB1 and CB2 with moderate selectivity for CB1. It is a derivative of HU-210 and represents a hybrid structure between the classical and nonclassical cannabinoid families. | |
| AM-926 | 2.2 | 4.3 | CB1 | A potent agonist at both CB1 and CB2 with moderate selectivity for CB1. It is a derivative of HU-210 and represents a hybrid structure between the classical and nonclassical cannabinoid families. | |||
| AM-938 | 1.2 | 0.3 | CB2 (4×) | 5.92 | 
|
A potent agonist at both CB1 and CB2. It is a derivative of HU-210 and represents a hybrid structure between the classical and nonclassical cannabinoid families. | |
| AM-1116 | 7.4 | A dimethylated stereoisomer of anandamide.[3] | |||||
| AM-1172 | An endocannabinoid analog specifically designed to be a potent and selective inhibitor of AEA uptake that is resistant to FAAH hydrolysis. | ||||||
| AM-1220 | 3.88 | 73.4 | CB1 (19×) | 4.73 | 
|
A potent and selective analgesic CB1 agonist (as racemate). The (R) enantiomer has around 1000× higher affinity for CB1 than (S) enantiomer.[8][9] | |
| AM-1221 | 52.3 | 0.28 | CB2 (187×) | 
|
A potent and selective CB2 agonist. | ||
| AM-1235 | 1.5 | 20.4 | CB1 (13×) | 
|
A moderately CB1 selective agonist.[10] | ||
| AM-1241 | 3.4 | CB2 (80×) | 
|
A potent and selective analgesic CB2 agonist.[11] | |||
| AM-1248 | CB1 | 
|
A moderately potent agonist with some selectivity for CB1, containing an unusual 3-(adamant-1-oyl) substitution on the indole ring. | ||||
| AM-1710 | Cannabilactone | CB2 (54×) | 
|
A CB2 selective cannabilactone.[12] Acts as a dual CB2 agonist / CB1 antagonist.[13] | |||
| AM-1714 | Cannabilactone | CB2 (490×) | 6.17 | 
|
A CB2 selective cannabilactone.[12] | ||
| AM-1902 | A nonclassical cannabinoid[14] | ||||||
| AM-2201 | 1.0 | 2.6 | CB1 | 5.18 | 
|
A potent agonist at both CB1 and CB2 with moderate selectivity for CB1. | |
| AM-2212 | 1.4 | 18.9 | CB1 | A potent agonist at both CB1 and CB2 with dodecal selectivity for CB1.[5] | |||
| AM-2213 | 3.0 | 30 | CB1 (10×) | A potent agonist at both CB1 and CB2.[5] | |||
| AM-2232 | 0.28 | 1.48 | 4.75 | 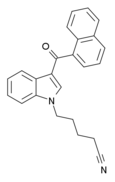
|
A potent agonist at both CB1 and CB2.[10] | ||
| AM-2233 | 1.8 | 2.2 | CB1 | 5.09 | 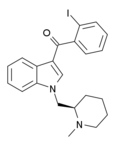
|
The (R) enantiomer is potent and selective CB1 agonist used in 131I radiolabelled form to map distribution of CB1 receptors in brain.[15][16][17][18][19][20] | |
| AM-2389 | 0.16 | CB1 (26×) | 6 | 
|
Classical cannabinoid derivative. | ||
| AM-3102 | 33000 | 26000 | An analog of oleoylethanolamide, the endogenous agonist for proliferator-activated receptor α (PPARα). It also acts as a weak cannabinoid agonist. | ||||
| AM-4030 | 0.7 | 8.6 | CB1 (12×) | 6.17 | 
|
A potent agonist at both CB1 and CB2, it is dodecally selective for CB1. It is a derivative of HU-210 and represents a hybrid structure between the classical and nonclassical cannabinoid families. | |
| AM-4054 | 2.2 | CB1 (40×) | A potent but slow-onset agonist.[21][22] | ||||
| AM-4056 | 0.041 | 6.51 | 
|
Another name for HU-243, it is a potent agonist at both the CB1 and CB2 receptors. | |||
| AM-4113 | CB1 | A CB1 selective neutral antagonist.[23] | |||||
| AM-6545 | CB1 | 4.06 | 
|
A peripherally selective silent antagonist of CB1 receptors. | |||
| AM-7438 | 
|
A potent agonist of CB1 and CB2 with reduced duration of action.[24] |
See also
[edit]- List of CP cannabinoids
- List of JWH cannabinoids
- List of HU cannabinoids
- List of miscellaneous designer cannabinoids
References
[edit]- ^ a b Lan, Ruoxi; Lu, Qian; Fan, Pusheng; Gatley, John; Volkow, Nora D.; Fernando, Susanthi R.; Pertwee, Roger; Makriyannis, Alexandros (1999). "Design and synthesis of the CB1 selective cannabinoid antagonist AM281: A potential human SPECT ligand". AAPS PharmSci. 1 (2): 39–45. doi:10.1208/ps010204. PMC 2761119. PMID 11741201.
- ^ "Alabama Senate Bill 333 - Controlled substances, Schedule I, additional synthetic controlled substances and analogue substances included in, trafficking in controlled substance analogues, requisite weight increased, Secs. 13A-12-231, 20-2-23 am'd". March 2014. Retrieved 28 September 2015.
- ^ a b c d Selwood, D. (2009). "The Cannabinoid Receptors. Edited by Patricia H. Reggio". ChemMedChem. 4 (11): 1949. doi:10.1002/cmdc.200900286.
- ^ Pacher, P.; Bátkai, S; Kunos, G (2006). "The Endocannabinoid System as an Emerging Target of Pharmacotherapy". Pharmacological Reviews. 58 (3): 389–462. doi:10.1124/pr.58.3.2. PMC 2241751. PMID 16968947.
- ^ a b c Hongfeng Deng. Design and synthesis of selective cannabinoid receptor ligands: Aminoalkylindole and other heterocyclic analogs. PhD Dissertation, University of Connecticut, 2000.
- ^ WO patent 200128557, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2001-06-07
- ^ Lu, D; Guo, J; Duclos, RI Jr; Bowman, AL; Makriyannis, A (Oct 2008). "Bornyl- and isobornyl-Delta8-tetrahydrocannabinols: a novel class of cannabinergic ligands". Journal of Medicinal Chemistry. 51 (20): 6393–9. doi:10.1021/jm8005299. PMC 3700413. PMID 18826296.
- ^ D'ambra, T. (1996). "C-Attached aminoalkylindoles: potent cannabinoid mimetics". Bioorganic & Medicinal Chemistry Letters. 6: 17–22. doi:10.1016/0960-894X(95)00560-G.
- ^ Willis, P. G.; Pavlova, O. A.; Chefer, S. I.; Vaupel, D. B.; Mukhin, A. G.; Horti, A. G. (2005). "Synthesis and Structure−Activity Relationship of a Novel Series of Aminoalkylindoles with Potential for Imaging the Neuronal Cannabinoid Receptor by Positron Emission Tomography". Journal of Medicinal Chemistry. 48 (18): 5813–22. doi:10.1021/jm0502743. PMID 16134948.
- ^ a b US patent 7241799, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2007-07-10
- ^ Poso, A.; Huffman, J. W. (2008). "Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands". British Journal of Pharmacology. 153 (2): 335–46. doi:10.1038/sj.bjp.0707567. PMC 2219524. PMID 17982473.
- ^ a b Khanolkar, AD; Lu, D; Ibrahim, M; Duclos, RI Jr; Thakur, GA; Malan, TP; Porreca, F; Veerappan, V; Tian, X; George, C; Parrish, DA; Papahatjis, DP; Makriyannis, A (Dec 2007). "Cannabilactones: a novel class of CB2 selective agonists with peripheral analgesic activity". Journal of Medicinal Chemistry. 50 (26): 6493–500. doi:10.1021/jm070441u. PMID 18038967.
- ^ Dhopeshwarkar, Amey; Murataeva, Natalia; Makriyannis, Alex; Straiker, Alex; Mackie, Ken (2016-12-07). "Two Janus Cannabinoids That Are Both CB2 Agonists and CB1 Antagonists". Journal of Pharmacology and Experimental Therapeutics. 360 (2). American Society for Pharmacology & Experimental Therapeutics (ASPET): 300–311. doi:10.1124/jpet.116.236539. ISSN 0022-3565. PMC 5267514. PMID 27927913.
- ^ Luk, T; Jin, W; Zvonok, A; Lu, D; Lin, XZ; Chavkin, C; Makriyannis, A; Mackie, K (Jun 2004). "Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist". Br J Pharmacol. 142 (3): 495–500. doi:10.1038/sj.bjp.0705792. PMC 1574962. PMID 15148260.
- ^ Deng H, Gifford AN, Zvonok AM, Cui G, Li X, Fan P, Deschamps JR, Flippen-Anderson JL, Gatley SJ, Makriyannis A (October 2005). "Potent cannabinergic indole analogues as radioiodinatable brain imaging agents for the CB1 cannabinoid receptor". Journal of Medicinal Chemistry. 48 (20): 6386–92. doi:10.1021/jm050135l. PMID 16190764.
- ^ Hanuš, L. R. O.; Mechoulam, R. (2005). "Cannabinoid chemistry: an overview". Cannabinoids as Therapeutics. Milestones in Drug Therapy MDT. p. 23. doi:10.1007/3-7643-7358-X_2. ISBN 3-7643-7055-6.
- ^ Shen CP, Xiao JC, Armstrong H, Hagmann W, Fong TM (February 2006). "F200A substitution in the third transmembrane helix of human cannabinoid CB1 receptor converts AM2233 from receptor agonist to inverse agonist". European Journal of Pharmacology. 531 (1–3): 41–6. doi:10.1016/j.ejphar.2005.12.026. PMID 16438957.
- ^ Dhawan, J.; Deng, H.; Gatley, S. J.; Makriyannis, A.; Akinfeleye, T.; Bruneus, M.; Dimaio, A. A.; Gifford, A. N. (2006). "Evaluation of the in vivo receptor occupancy for the behavioral effects of cannabinoids using a radiolabeled cannabinoid receptor agonist, R-[125/131I]AM2233". Synapse. 60 (2): 93–101. doi:10.1002/syn.20277. PMID 16715483. S2CID 21269336.
- ^ Leung K (Dec 12, 2006). "R-2-[131I]Iodophenyl-(1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl)methanone". Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. PMID 20641836.
- ^ Pei, Y.; et al. (2008). "Ligand-Binding Architecture of Human CB2 Cannabinoid Receptor: Evidence for Receptor Subtype-Specific Binding Motif and Modeling GPCR Activation". Chemistry & Biology. 15 (11): 1207–1219. doi:10.1016/j.chembiol.2008.10.011. PMC 3700404. PMID 19022181.
- ^ [Paronis CA, Thakur GA, Vemuri K, Makriyannis A, Bergman J. Effects of a Selective Cannabinoid Agonist and Antagonist on Body Temperature in Rats. The FASEB Journal. April 2007 21 (Meeting Abstract Supplement) A409. http://www.fasebj.org/cgi/content/meeting_abstract/21/5/A409]
- ^ Paronis, C. A.; Thakur, G. A.; Bajaj, S.; Nikas, S. P.; Vemuri, V. K.; Makriyannis, A.; Bergman, J. (2012). "Diuretic effects of cannabinoids". Journal of Pharmacology and Experimental Therapeutics. 344 (1): 8–14. doi:10.1124/jpet.112.199331. PMC 3533417. PMID 23019138.
- ^ Seely, KA; Prather, PL; James, LP; Moran, JH (Feb 2011). "Marijuana-based drugs: innovative therapeutics or designer drugs of abuse?". Molecular Interventions. 11 (1): 36–51. doi:10.1124/mi.11.1.6. PMC 3139381. PMID 21441120.
- ^ Nikas SP, Sharma R, Paronis CA, Kulkarni S, Thakur GA, Hurst D, Wood JT, Gifford RS, Rajarshi G, Liu Y, Raghav JG, Guo JJ, Järbe TU, Reggio PH, Bergman J, Makriyannis A. Probing the carboxyester side chain in controlled deactivation (-)-δ(8)-tetrahydrocannabinols. J Med Chem. 2015 Jan 22;58(2):665-81. doi:10.1021/jm501165d PMID 25470070
