ICI-118,551
| |
| Names | |
|---|---|
| IUPAC name
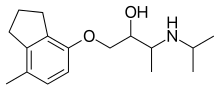
3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| MeSH | ICI+118551 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C17H27NO2 | |
| Molar mass | 277.402 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
ICI-118,551 is a selective β2 adrenergic receptor (adrenoreceptor) antagonist or beta blocker.[1][2] ICI binds to the β2 subtype with at least 100 times greater affinity than β1 or β3, the two other known subtypes of the beta adrenoceptor.[3][4] The compound was developed by Imperial Chemical Industries, which was acquired by AkzoNobel in 2008.
ICI-118,551 has no known therapeutic use in humans although it has been used widely in research to understand the action of the β2 adrenergic receptor, as few other specific antagonists for this receptor are known.[5] ICI-118,551 has been used in pre-clinical studies using murine models.[6][7][8] When dissolved in saline, the compound crosses the blood–brain barrier. Common systemic doses used in rodent research are 0.5 or 1 mg/kg although efficacy has been demonstrated at doses as low as 0.0001 mg/kg (100 ng/kg) in rhesus monkeys.[9] Doses up to 20 mg/kg have been used without toxicity. At room temperature in saline, the ICI 118,551 hydrochloride is soluble to at least 2.5 mg/mL.
References
[edit]- ^ Hillman KL, Doze VA, Porter JE (August 2005). "Functional characterization of the beta-adrenergic receptor subtypes expressed by CA1 pyramidal cells in the rat hippocampus". The Journal of Pharmacology and Experimental Therapeutics. 314 (2): 561–7. doi:10.1124/jpet.105.084947. PMID 15908513. S2CID 12446381.
- ^ Summerhill S, Stroud T, Nagendra R, Perros-Huguet C, Trevethick M (2008). "A cell-based assay to assess the persistence of action of agonists acting at recombinant human beta(2) adrenoceptors". Journal of Pharmacological and Toxicological Methods. 58 (3): 189–97. doi:10.1016/j.vascn.2008.06.003. PMID 18652905.
- ^ Mauriège P, De Pergola G, Berlan M, Lafontan M (May 1988). "Human fat cell beta-adrenergic receptors: beta-agonist-dependent lipolytic responses and characterization of beta-adrenergic binding sites on human fat cell membranes with highly selective beta 1-antagonists". Journal of Lipid Research. 29 (5): 587–601. doi:10.1016/S0022-2275(20)38502-3. PMID 2900871.
- ^ Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg AD (September 1989). "Molecular characterization of the human beta 3-adrenergic receptor". Science. 245 (4922): 1118–21. Bibcode:1989Sci...245.1118E. doi:10.1126/science.2570461. PMID 2570461.
- ^ Ruffolo Jr RR, ed. (1995). Adrenoceptors: Structure, Function. and Pharmacology. Luxembourg: Harwood Academic Publisher.
- ^ Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, et al. (21 December 2017). "Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice". Oncoimmunology. 7 (3): e1405205. doi:10.1080/2162402X.2017.1405205. PMC 5790362. PMID 29399407.
- ^ Branca C, Wisely EV, Hartman LK, Caccamo A, Oddo S (December 2014). "Administration of a selective β2 adrenergic receptor antagonist exacerbates neuropathology and cognitive deficits in a mouse model of Alzheimer's disease". Neurobiology of Aging. 35 (12): 2726–2735. doi:10.1016/j.neurobiolaging.2014.06.011. PMC 4252846. PMID 25034342.
- ^ Nagaraja S, Iyer S, Liu X, Eichberg J, Bond RA (July 1999). "Treatment with inverse agonists enhances baseline atrial contractility in transgenic mice with chronic beta2-adrenoceptor activation". British Journal of Pharmacology. 127 (5): 1099–104. doi:10.1038/sj.bjp.0702645. PMC 1566118. PMID 10455254.
- ^ Ramos BP, Arnsten AF (March 2007). "Adrenergic pharmacology and cognition: focus on the prefrontal cortex". Pharmacology & Therapeutics. 113 (3): 523–36. doi:10.1016/j.pharmthera.2006.11.006. PMC 2151919. PMID 17303246.
