Phenylpropanolamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Many[1][2] |
| Other names | PPA; Norephedrine; (1RS,2SR)-Phenylpropanolamine; dl-Norephedrine; (±)-Norephedrine; (1RS,2SR)-α-Methyl-β-hydroxyphenethylamine; (1RS,2SR)-β-Hydroxyamphetamine |
| AHFS/Drugs.com | Multum Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High[4] |
| Protein binding | 20%[5][4] |
| Metabolism | Minimal (3–4%)[5][7][4] |
| Metabolites | • Hippuric acid (~4%)[4][5] • 4-Hydroxynorephedrine (≤1%)[5][4] |
| Onset of action | Oral: 15–30 minutes[4][6] |
| Elimination half-life | 4 (3.7–4.9) hours[4][6][7][8] |
| Duration of action | Oral: 3 hours[4][6] |
| Excretion | Urine: 90% (unchanged)[6][4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.349 |
| Chemical and physical data | |
| Formula | C9H13NO |
| Molar mass | 151.209 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Phenylpropanolamine (PPA), sold under many brand names, is a sympathomimetic agent which is used as a decongestant and appetite suppressant.[9][1][10][11] It was previously commonly used in prescription and over-the-counter cough and cold preparations. The medication is taken by mouth.[4][12]
Side effects of PPA include increased heart rate and blood pressure, among others.[13][14][15][12] Rarely, PPA has been associated with hemorrhagic stroke.[11][16][13] PPA acts as a norepinephrine releasing agent, thereby indirectly activating adrenergic receptors.[17][18][19] As such, it is an indirectly acting sympathomimetic.[17][18][19][10] It was previously thought to act as a mixed acting sympathomimetic with additional direct agonist actions on adrenergic receptors, but this proved not to be the case.[17][18][19] Chemically, PPA is a substituted amphetamine and is closely related to ephedrine, pseudoephedrine, amphetamine, and cathinone.[20][21][22][11] It is most commonly a racemic mixture of the (1R,2S)- and (1S,2R)-enantiomers of β-hydroxyamphetamine and is also known as dl-norephedrine.[21][9][1]
PPA was first synthesized around 1910 and its effects on blood pressure were first characterized around 1930.[21][11] It was introduced for medical use by the 1930s.[23][11] The medication was withdrawn from many markets starting in 2000 following findings that it was associated with increased risk of hemorrhagic stroke.[23][11] It was previously available both over-the-counter and by prescription.[23][2][24][25] PPA remains available for medical and/or veterinary use in some countries.[2]
Medical uses
[edit]PPA is used as a decongestant to treat nasal congestion.[13][14] It has also been used to suppress appetite and promote weight loss in the treatment of obesity and has shown effectiveness for this indication.[26][27][28]
Available forms
[edit]PPA was previously available over-the-counter and in certain combination forms by prescription in the United States.[24][25] However, these forms have all been discontinued.[24][25][2] PPA remains available in certain other countries.[2]
Side effects
[edit]PPA produces sympathomimetic effects and can cause side effects such as increased heart rate and blood pressure.[13][14][15][12] It has been associated rarely with incidence of hemorrhagic stroke.[23][16][13]
Certain drugs increase the chances of déjà vu occurring in the user, resulting in a strong sensation that an event or experience currently being experienced has already been experienced in the past. Some pharmaceutical drugs, when taken together, have also been implicated in the cause of déjà vu.[29] The Journal of Clinical Neuroscience reported the case of an otherwise healthy male who started experiencing intense and recurrent sensations of déjà vu upon taking the drugs amantadine and PPA together to relieve flu symptoms.[30] He found the experience so interesting that he completed the full course of his treatment and reported it to the psychologists to write up as a case study. Because of the dopaminergic action of the drugs and previous findings from electrode stimulation of the brain,[31] it was speculated that déjà vu occurs as a result of hyperdopaminergic action in the mesial temporal areas of the brain.
Interactions
[edit]There has been very little research on drug interactions with PPA.[4] In one study, PPA taken with caffeine was found to quadruple caffeine levels.[4] In another study, PPA reduced theophylline clearance by 50%.[4]
Pharmacology
[edit]Pharmacodynamics
[edit]PPA acts primarily as a selective norepinephrine releasing agent.[19] It also acts as a dopamine releasing agent with around 10-fold lower potency.[19] The stereoisomers of the drug have only weak or negligible affinity for α- and β-adrenergic receptors.[19]
| Compound | NE | DA | 5-HT | Ref | ||
|---|---|---|---|---|---|---|
| Dextroamphetamine (S(+)-amphetamine) | 6.6–7.2 | 5.8–24.8 | 698–1765 | [33][34] | ||
| S(–)-Cathinone | 12.4 | 18.5 | 2366 | [19] | ||
| Ephedrine ((–)-ephedrine) | 43.1–72.4 | 236–1350 | >10000 | [33] | ||
| (+)-Ephedrine | 218 | 2104 | >10000 | [33][19] | ||
| Dextromethamphetamine (S(+)-methamphetamine) | 12.3–13.8 | 8.5–24.5 | 736–1291.7 | [33][35] | ||
| Levomethamphetamine (R(–)-methamphetamine) | 28.5 | 416 | 4640 | [33] | ||
| (+)-Phenylpropanolamine ((+)-norephedrine) | 42.1 | 302 | >10000 | [19] | ||
| (–)-Phenylpropanolamine ((–)-norephedrine) | 137 | 1371 | >10000 | [19] | ||
| Cathine ((+)-norpseudoephedrine) | 15.0 | 68.3 | >10000 | [19] | ||
| (–)-Norpseudoephedrine | 30.1 | 294 | >10000 | [19] | ||
| (–)-Pseudoephedrine | 4092 | 9125 | >10000 | [19] | ||
| Pseudoephedrine ((+)-pseudoephedrine) | 224 | 1988 | >10000 | [19] | ||
| The smaller the value, the more strongly the substance releases the neurotransmitter. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. | ||||||
PPA was originally thought to act as a direct agonist of adrenergic receptors and hence to act as a mixed acting sympathomimetic,[21][22] However, PPA was subsequently found to show only weak or negligible affinity for these receptors and has been instead characterized as exclusively an indirectly acting sympathomimetic.[10][17][18][19] It acts by inducing norepinephrine release and thereby indirectly activating adrenergic receptors.[17][18][19]
Many sympathetic hormones and neurotransmitters are based on the phenethylamine skeleton, and function generally in "fight or flight" type responses, such as increasing heart rate, blood pressure, dilating the pupils, increased energy, drying of mucous membranes, increased sweating, and a significant number of additional effects.[citation needed]
PPA has relatively low potency as a sympathomimetic.[21] It is about 100 to 200 times less potent than epinephrine (adrenaline) or norepinephrine (noradrenaline) in its sympathomimetic effects, although responses are variable depending on tissue.[21]
Pharmacokinetics
[edit]Absorption
[edit]PPA is readily- and well-absorbed with oral administration.[6][7][5] Immediate-release forms of the drug reached peak levels about 1.5 hours (range 1.0 to 2.3 hours) following administration.[4][7] Conversely, extended-release forms of PPA reach peak levels after 3.0 to 4.5 hours.[4] The pharmacokinetics of PPA are linear across an oral dose range of 25 to 100 mg.[4] Steady-state levels of PPA are achieved within 12 hours when the drug is taken once every 4 hours.[4] There is 62% accumulation of PPA at steady state in terms of peak levels, whereas area-under-the-curve levels are not increased with steady state.[4]
Distribution
[edit]The volume of distribution of PPA is 3.0 to 4.5 L/kg.[4] Levels of PPA in the brain are about 40% of those in the heart and 20% of those in the lungs.[6] The hydroxyl group of PPA at the β carbon increases its hydrophilicity, reduces its permeation through the blood–brain barrier, and limits its central nervous system (CNS) effects.[6] Hence, PPA crosses into the brain only to some extent, has only weak CNS effects, and most of its effects are peripheral.[14][6][5][21] In any case, PPA can produce amphetamine-like psychostimulant effects at very high doses.[21][6][5] PPA is more lipophilic than structurally related sympathomimetics with hydroxyl groups on the phenyl ring like epinephrine (adrenaline) and phenylephrine and has greater brain permeability than these agents.[5][22]
The plasma protein binding of PPA is approximately 20%.[5][4] However, it has been said that no recent studies have substantiated this value.[4]
Metabolism
[edit]PPA is not substantially metabolized.[7][5] It also does not undergo significant first-pass metabolism.[7] Only about 3 to 4% of an oral dose of PPA is metabolized.[5] Metabolites include hippuric acid (via oxidative deamination of the side chain) and 4-hydroxynorephedrine (via para-hydroxylation).[4][5][6] The methyl group at the α carbon of PPA blocks metabolism by monoamine oxidases (MAOs).[6][5][14] PPA is also not a substrate of catechol O-methyltransferase.[14] The hydroxyl group at the β carbon of PPA also helps to increase metabolic stability.[5]
Elimination
[edit]Approximately 90% of a dose of PPA is excreted in the urine unchanged within 24 hours.[4][6][7][5] About 4% of excreted material is in the form of metabolites.[4]
The elimination half-life of immediate-release PPA is about 4 hours, with a range in different studies of 3.7 to 4.9 hours.[6][7][4] The half-life of extended-release PPA has ranged from 4.3 to 5.8 hours.[4]
The elimination of PPA is dependent on urinary pH.[4][5] At a more acidic urinary pH, the elimination of PPA is accelerated and its half-life and duration are shortened, whereas at more basic urinary pH, the elimination of PPA is reduced and its half-life and duration are extended.[5] [4] Urinary acidifying agents like ascorbic acid and ammonium chloride can increase the excretion of and thereby reduce exposure to amphetamines including PPA, whereas urinary alkalinizing agents including antacids like sodium bicarbonate as well as acetazolamide can reduce the excretion of these agents and thereby increase exposure to them.[36][5][37]
Total body clearance of PPA has been reported to be 0.546 L/h/kg, while renal clearance was 0.432 L/h/kg.[4]
Miscellaneous
[edit]As PPA is not extensively metabolized, it would probably not be affected by hepatic impairment.[4] Conversely, there is likely to be accumulation of PPA with renal impairment due to its dependence on urinary excretion.[4]
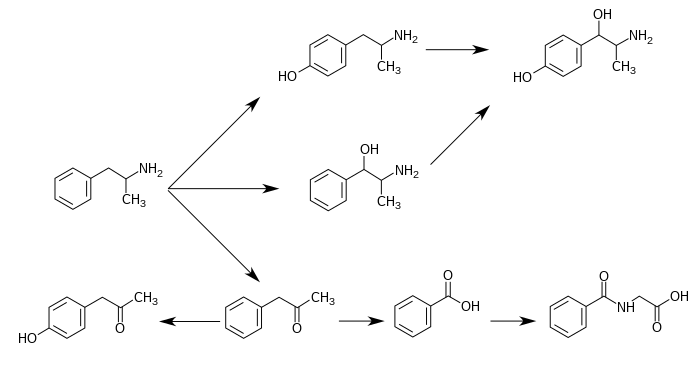
Norephedrine is a minor metabolite of amphetamine and methamphetamine, as shown below.[4] It is also a minor metabolite of ephedrine and a major metabolite of cathinone.[4][6][5]
Metabolic pathways of amphetamine in humans[sources 1]
|
Chemistry
[edit]
PPA, also known as (1RS,2SR)-α-methyl-β-hydroxyphenethylamine or as (1RS,2SR)-β-hydroxyamphetamine, is a substituted phenethylamine and amphetamine derivative.[9][20][49] It is closely related to the cathinones (β-ketoamphetamines).[20] β-Hydroxyamphetamine exists as four stereoisomers, which include d- (dextrorotatory) and l-norephedrine (levorotatory), and d- and l-norpseudoephedrine.[49][10] d-Norpseudoephedrine is also known as cathine,[9][49] and is found naturally in Catha edulis (khat).[50] Pharmaceutical drug preparations of PPA have varied in their stereoisomer composition in different countries, which may explain differences in misuse and side effect profiles.[10] In any case, racemic dl-norephedrine, or (1RS,2SR)-phenylpropanolamine, appears to be the most commonly used formulation of PPA pharmaceutically.[21][9][1] Analogues of PPA include ephedrine, pseudoephedrine, amphetamine, methamphetamine, and cathinone.[20]
PPA, structurally, is in the substituted phenethylamine class, consisting of a cyclic benzene or phenyl group, a two carbon ethyl moiety, and a terminal nitrogen, hence the name phen-ethyl-amine.[51] The methyl group on the alpha carbon (the first carbon before the nitrogen group) also makes this compound a member of the substituted amphetamine class.[51] Ephedrine is the N-methyl analogue of PPA.
Exogenous compounds in this family are degraded too rapidly by monoamine oxidase to be active at all but the highest doses.[51] However, the addition of the α-methyl group allows the compound to avoid metabolism and confer an effect.[51] In general, N-methylation of primary amines increases their potency, whereas β-hydroxylation decreases CNS activity, but conveys more selectivity for adrenergic receptors.[51]
PPA is a small-molecule compound with the molecular formula C9H13NO and a molecular weight of 151.21 g/mol.[52][8] It has an experimental log P of 0.67, while its predicted log P values range from 0.57 to 0.89.[52][8] The compound is relatively lipophilic,[5] but is also more hydrophilic than other amphetamines.[6] The lipophilicity of amphetamines is closely related to their brain permeability.[53] For comparison to PPA, the experimental log P of methamphetamine is 2.1,[54] of amphetamine is 1.8,[55][54] of ephedrine is 1.1,[56] of pseudoephedrine is 0.7,[57] of phenylephrine is -0.3,[58] and of norepinephrine is -1.2.[59] Methamphetamine has high brain permeability,[54] whereas phenylephrine and norepinephrine are peripherally selective drugs.[60][61] The optimal log P for brain permeation and central activity is about 2.1 (range 1.5–2.7).[62]
PPA has been used pharmaceutically exclusively as the hydrochloride salt.[9][1]
History
[edit]PPA was first synthesized in the early 20th century, in or around 1910.[21][11] It was patented as a mydriatic in 1913.[21] The pressor effects of PPA were characterized in the late 1920s and the 1930s.[21] PPA was first introduced for medical use by the 1930s.[23][11]
In the United States, PPA is no longer sold due to an increased risk of haemorrhagic stroke.[16] In a few countries in Europe, however, it is still available either by prescription or sometimes over-the-counter. In Canada, it was withdrawn from the market on 31 May 2001.[63] It was voluntarily withdrawn from the Australian market by July 2001.[64] In India, human use of PPA and its formulations was banned on 10 February 2011,[65] but the ban was overturned by the judiciary in September 2011.[66]
Society and culture
[edit]Names
[edit]Phenylpropanolamine is the generic name of the drug and its INN, BAN, and DCF, while phenylpropanolamine hydrochloride is its USAN and BANM in the case of the hydrochloride salt.[9][1][10][2] It is also known by the synonym norephedrine.[9][1][2]
Brand names of PPA have included Acutrim, Appedrine, Capton Diet, Control, Dexatrim, Emagrin Plus A.P., Glifentol, Kontexin, Merex, Monydrin, Mydriatine, Prolamine, Propadrine, Propagest, Recatol, Rinexin, Tinaroc, and Westrim, among many others.[9][1][2] It has also been used in combinations under brand names including Allerest, Demazin, Dimetapp, and Sinarest, among others.[1][2]
Availability
[edit]PPA remains available for medical and veterinary use in certain countries.[1][2]
Exercise and sports
[edit]There has been interest in PPA as a performance-enhancing drug in exercise and sports.[67] However, clinical studies suggest that PPA is not effective in this regard.[67][6] PPA is not on the World Anti-Doping Agency (WADA) list of prohibited substances as of 2024.[68]
Legal status
[edit]In Sweden, PPA is still available in prescription decongestants;[69] PPA is also still available in Germany. It is used in some polypill medications like Wick DayMed capsules.
In the United Kingdom, PPA was available in many "all in one" cough and cold medications which usually also feature paracetamol or another analgesic and caffeine and could also be purchased on its own; however, it is no longer approved for human use. A European Category 1 Licence is required to purchase PPA for academic use.
In the United States, the Food and Drug Administration (FDA) issued a public health advisory[70] against the use of the drug in November 2000. In this advisory, the FDA requested but did not require that all drug companies discontinue marketing products containing PPA. The agency estimates that PPA caused between 200 and 500 strokes per year among 18-to-49-year-old users. In 2005, the FDA removed PPA from over-the-counter sale and removed its "generally recognized as safe and effective" (GRASE) status.[71] Under the 2020 CARES Act, it requires FDA approval before it can be marketed again effectively banning the drug even as a prescription drug.[72]
Because of its potential use in amphetamine manufacture, PPA is controlled by the Combat Methamphetamine Epidemic Act of 2005. It is still available for veterinary use in dogs, however, as a treatment for urinary incontinence.
Internationally, an item on the agenda of the 2000 Commission on Narcotic Drugs session called for including the stereoisomer norephedrine in Table I of United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[73]
Drugs containing PPA were banned in India on 27 January 2011.[74] On 13 September 2011, Madras High Court revoked a ban on manufacture and sale of pediatric drugs PPA and nimesulide.[75]
Veterinary use
[edit]PPA remains available for use in veterinary medicine.[25] It is used to control urinary incontinence in dogs.[76][77]
Notes
[edit]- ^ 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[39][44] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[44][46] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[47][48]
Reference notes
[edit]References
[edit]- ^ a b c d e f g h i j Schweizerischer Apotheker-Verein (2000). Index Nominum 2000: International Drug Directory. Medpharm Scientific Publishers. p. 828. ISBN 978-3-88763-075-1. Retrieved 1 August 2024.
- ^ a b c d e f g h i j "Phenylpropanolamine". 24 March 2015. Archived from the original on 6 May 2015. Retrieved 1 August 2024.
{{cite web}}: CS1 maint: unfit URL (link) - ^ Anvisa (24 July 2023). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 25 July 2023). Archived from the original on 27 August 2023. Retrieved 27 August 2023.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Kanfer I, Dowse R, Vuma V (1993). "Pharmacokinetics of oral decongestants". Pharmacotherapy. 13 (6 Pt 2): 116S–128S, discussion 143S–146S. PMID 7507589.
- ^ a b c d e f g h i j k l m n o p q r s t Chua SS, Benrimoj SI, Triggs EJ (1989). "Pharmacokinetics of non-prescription sympathomimetic agents". Biopharm Drug Dispos. 10 (1): 1–14. doi:10.1002/bdd.2510100102. PMID 2647163.
- ^ a b c d e f g h i j k l m n o p Bouchard R, Weber AR, Geiger JD (July 2002). "Informed decision-making on sympathomimetic use in sport and health". Clin J Sport Med. 12 (4): 209–224. doi:10.1097/00042752-200207000-00003. PMID 12131054.
- ^ a b c d e f g h Gentile DA, Friday GA, Skoner DP (2000). "Management of allergic rhinitis: antihistamines and decongestants". Immunology and Allergy Clinics of North America. 20 (2): 355–368. doi:10.1016/S0889-8561(05)70152-1.
- ^ a b c "Phenylpropanolamine: Uses, Interactions, Mechanism of Action". DrugBank Online. 29 June 2018. Retrieved 1 August 2024.
- ^ a b c d e f g h i Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 71. ISBN 978-1-4757-2085-3. Retrieved 1 August 2024.
- ^ a b c d e f Morton IK, Hall JM (2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. p. 219. ISBN 978-94-011-4439-1. Retrieved 1 August 2024.
- ^ a b c d e f g h Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ (2006). "Safety of drug therapies used for weight loss and treatment of obesity". Drug Saf. 29 (4): 277–302. doi:10.2165/00002018-200629040-00001. PMID 16569079.
- ^ a b c Salerno SM, Jackson JL, Berbano EP (August 2005). "The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature". J Hum Hypertens. 19 (8): 643–652. doi:10.1038/sj.jhh.1001869. PMID 15944721.
- ^ a b c d e Johnson DA, Hricik JG (1993). "The pharmacology of alpha-adrenergic decongestants". Pharmacotherapy. 13 (6 Pt 2): 110S–115S, discussion 143S–146S. PMID 7507588.
- ^ a b c d e f O'Donnell SR (March 1995). "Sympathomimetic vasoconstrictors as nasal decongestants". Med J Aust. 162 (5): 264–267. doi:10.5694/j.1326-5377.1995.tb139882.x. PMID 7534374.
- ^ a b Aaron CK (August 1990). "Sympathomimetics". Emerg Med Clin North Am. 8 (3): 513–526. PMID 2201518.
- ^ a b c Yoon BW, Bae HJ, Hong KS, Lee SM, Park BJ, Yu KH, et al. (January 2007). "Phenylpropanolamine contained in cold remedies and risk of hemorrhagic stroke". Neurology. 68 (2): 146–149. doi:10.1212/01.wnl.0000250351.38999.f2. PMID 17210897. S2CID 211233331.
- ^ a b c d e f Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Curr Top Med Chem. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961.
- ^ a b c d e Rothman RB, Baumann MH (December 2005). "Targeted screening for biogenic amine transporters: potential applications for natural products". Life Sci. 78 (5): 512–518. doi:10.1016/j.lfs.2005.09.001. PMID 16202429.
- ^ a b c d e f g h i j k l m n o p Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, et al. (October 2003). "In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates". J Pharmacol Exp Ther. 307 (1): 138–145. doi:10.1124/jpet.103.053975. PMID 12954796.
- ^ a b c d Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 643–. ISBN 978-0-7817-6879-5.
- ^ a b c d e f g h i j k l Johnson DA (1991). "Pharmacology and safety of phenylpropanolamine". Drug Development Research. 22 (3): 197–207. doi:10.1002/ddr.430220302. ISSN 0272-4391.
- ^ a b c Bravo EL (March 1988). "Phenylpropanolamine and other over-the-counter vasoactive compounds". Hypertension. 11 (3 Pt 2): II7–10. doi:10.1161/01.hyp.11.3_pt_2.ii7. PMID 3280497.
- ^ a b c d e Mersfelder TL (March 2001). "Phenylpropanolamine and stroke: the study, the FDA ruling, the implications". Cleve Clin J Med. 68 (3): 208–9, 213–9, 223. doi:10.3949/ccjm.68.3.208. PMID 11263849.
- ^ a b c "Drugs@FDA: FDA-Approved Drugs". accessdata.fda.gov. Food and Drug Administration. Retrieved 1 August 2024.
- ^ a b c d "Search Results for phenylpropanolamine". DailyMed. Retrieved 1 August 2024.
- ^ Coulter AA, Rebello CJ, Greenway FL (July 2018). "Centrally Acting Agents for Obesity: Past, Present, and Future". Drugs. 78 (11): 1113–1132. doi:10.1007/s40265-018-0946-y. PMC 6095132. PMID 30014268.
- ^ Ioannides-Demos LL, Proietto J, McNeil JJ (2005). "Pharmacotherapy for obesity". Drugs. 65 (10): 1391–418. doi:10.2165/00003495-200565100-00006. PMID 15977970.
- ^ Greenway F, Herber D, Raum W, Herber D, Morales S (July 1999). "Double-blind, randomized, placebo-controlled clinical trials with non-prescription medications for the treatment of obesity". Obes Res. 7 (4): 370–378. doi:10.1002/j.1550-8528.1999.tb00420.x. PMID 10440593.
- ^ Taiminen T, Jääskeläinen SK (September 2001). "Intense and recurrent déjà vu experiences related to amantadine and phenylpropanolamine in a healthy male". Journal of Clinical Neuroscience. 8 (5): 460–462. doi:10.1054/jocn.2000.0810. PMID 11535020. S2CID 6733989.
- ^ Taiminen T, Jääskeläinen SK (September 2001). "Intense and recurrent déjà vu experiences related to amantadine and phenylpropanolamine in a healthy male". Journal of Clinical Neuroscience. 8 (5): 460–462. doi:10.1054/jocn.2000.0810. PMID 11535020. S2CID 6733989.
- ^ Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E (February 1994). "Anatomical origin of déjà vu and vivid 'memories' in human temporal lobe epilepsy". Brain. 117 (Pt 1): 71–90. doi:10.1093/brain/117.1.71. PMID 8149215.
- ^ Rothman RB, Baumann MH (2003). "Monoamine transporters and psychostimulant drugs". Eur. J. Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ a b c d e Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ^ Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. (2013). "Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products". Neuropsychopharmacology. 38 (4): 552–562. doi:10.1038/npp.2012.204. PMC 3572453. PMID 23072836.
- ^ Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. (April 2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ Głowacka K, Wiela-Hojeńska A (May 2021). "Pseudoephedrine—Benefits and Risks". Int J Mol Sci. 22 (10): 5146. doi:10.3390/ijms22105146. PMC 8152226. PMID 34067981.
- ^ Patrick KS, Markowitz JS (1997). "Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder". Human Psychopharmacology: Clinical and Experimental. 12 (6): 527–546. doi:10.1002/(SICI)1099-1077(199711/12)12:6<527::AID-HUP932>3.0.CO;2-U. ISSN 0885-6222.
- ^ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ^ a b Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. doi:10.1016/S0021-9258(19)43051-2. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- ^ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ^ a b c Sjoerdsma A, von Studnitz W (April 1963). "Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate". British Journal of Pharmacology and Chemotherapy. 20 (2): 278–284. doi:10.1111/j.1476-5381.1963.tb01467.x. PMC 1703637. PMID 13977820.
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
- ^ Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation". Expert Opinion on Drug Metabolism & Toxicology. 9 (9): 1139–1153. doi:10.1517/17425255.2013.796929. PMID 23650932. S2CID 23738007.
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
- ^ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circulation Research. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201. S2CID 28641000.
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study, hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
- ^ Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine". Neuropharmacology. 13 (12): 1187–1190. doi:10.1016/0028-3908(74)90069-0. PMID 4457764.
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway, p-hydroxyamphetamine (POH) and p-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the p-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN. - ^ Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems". Journal of Pharmacology and Experimental Therapeutics. 251 (3): 901–908. PMID 2600821.
The metabolism of p-OHA to p-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert p-OHA to p-OHNor after intraventricular administration.
- ^ a b c King LA (2009). Forensic Chemistry of Substance Misuse: A Guide to Drug Control. Royal Society of Chemistry. pp. 53–. ISBN 978-0-85404-178-7.
- ^ Balint EE, Falkay G, Balint GA (2009). "Khat - a controversial plant". Wiener Klinische Wochenschrift. 121 (19–20): 604–614. doi:10.1007/s00508-009-1259-7. PMID 19921126. S2CID 22816940.
- ^ a b c d e Westfall DP, Westfall TC (2010). "Chapter 12: Adrenergic Agonists and Antagonists: CLASSIFICATION OF SYMPATHOMIMETIC DRUGS". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 9780071624428.
CHEMISTRY AND STRUCTURE-ACTIVITY RELATIONSHIP OF SYMPATHOMIMETIC AMINES
β-Phenylethylamine (Table 12–1) can be viewed as the parent compound of the sympathomimetic amines, consisting of a benzene ring and an ethylamine side chain. The structure permits substitutions to be made on the aromatic ring, the α- and β-carbon atoms, and the terminal amino group to yield a variety of compounds with sympathomimetic activity. ...N-methylation increases the potency of primary amines ...
Substitution on the α-Carbon Atom
This substitution blocks oxidation by MAO, greatly prolonging the duration of action of non-catecholamines because their degradation depends largely on the action of this enzyme. The duration of action of drugs such as ephedrine or amphetamine is thus measured in hours rather than in minutes. Similarly, compounds with an α-methyl substituent persist in the nerve terminals and are more likely to release NE from storage sites. Agents such as metaraminol exhibit a greater degree of indirect sympathomimetic activity.
Substitution on the β-Carbon Atom
Substitution of a hydroxyl group on the β carbon generally decreases actions within the CNS, largely because it lowers lipid solubility. However, such substitution greatly enhances agonist activity at both α- and β- adrenergic receptors. Although ephedrine is less potent than methamphetamine as a central stimulant, it is more powerful in dilating bronchioles and increasing blood pressure and heart rate. - ^ a b "Phenylpropanolamine". PubChem. Retrieved 1 August 2024.
- ^ Bharate SS, Mignani S, Vishwakarma RA (December 2018). "Why Are the Majority of Active Compounds in the CNS Domain Natural Products? A Critical Analysis". J Med Chem. 61 (23): 10345–10374. doi:10.1021/acs.jmedchem.7b01922. PMID 29989814.
- ^ a b c Schep LJ, Slaughter RJ, Beasley DM (August 2010). "The clinical toxicology of metamfetamine". Clin Toxicol (Phila). 48 (7): 675–694. doi:10.3109/15563650.2010.516752. PMID 20849327.
Metamfetamine acts in a manner similar to amfetamine, but with the addition of the methyl group to the chemical structure. It is more lipophilic (Log p value 2.07, compared with 1.76 for amfetamine),4 thereby enabling rapid and extensive transport across the blood–brain barrier.19
- ^ "Amphetamine". PubChem. Retrieved 26 July 2024.
- ^ "Ephedrine". PubChem. Retrieved 26 July 2024.
- ^ "Pseudoephedrine". PubChem. Retrieved 1 August 2024.
- ^ "Phenylephrine". PubChem. Retrieved 21 July 2024.
- ^ "Norepinephrine". PubChem. Retrieved 26 July 2024.
- ^ Eccles R (January 2007). "Substitution of phenylephrine for pseudoephedrine as a nasal decongeststant. An illogical way to control methamphetamine abuse". British Journal of Clinical Pharmacology. 63 (1): 10–14. doi:10.1111/j.1365-2125.2006.02833.x. PMC 2000711. PMID 17116124.
- ^ Froese L, Dian J, Gomez A, Unger B, Zeiler FA (October 2020). "The cerebrovascular response to norepinephrine: A scoping systematic review of the animal and human literature". Pharmacol Res Perspect. 8 (5): e00655. doi:10.1002/prp2.655. PMC 7510331. PMID 32965778.
- ^ Pajouhesh H, Lenz GR (October 2005). "Medicinal chemical properties of successful central nervous system drugs". NeuroRx. 2 (4): 541–553. doi:10.1602/neurorx.2.4.541. PMC 1201314. PMID 16489364.
Lipophilicity was the first of the descriptors to be identified as important for CNS penetration. Hansch and Leo54 reasoned that highly lipophilic molecules will partitioned into the lipid interior of membranes and will be retained there. However, ClogP correlates nicely with LogBBB with increasing lipophilicity increasing brain penetration. For several classes of CNS active substances, Hansch and Leo54 found that blood-brain barrier penetration is optimal when the LogP values are in the range of 1.5-2.7, with the mean value of 2.1. An analysis of small drug-like molecules suggested that for better brain permeation46 and for good intestinal permeability55 the LogD values need to be greater than 0 and less than 3. In comparison, the mean value for ClogP for the marketed CNS drugs is 2.5, which is in good agreement with the range found by Hansch et al.22
- ^ "Advisories, Warnings and Recalls – 2001". Health Canada. 7 January 2009. Archived from the original on 3 May 2010.
- ^ "Alert Phenylpropanolamine". Therapeutic Goods Administration. 7 March 2006. Retrieved 31 December 2018.
- ^ "Drugs Banned In India". Dte.GHS, Ministry of Health and Family Welfare, Government of India. Central Drugs Standard Control Organization. Archived from the original on 13 October 2013. Retrieved 7 January 2014.
- ^ "Madras High Court revokes ban on manufacture and sale of paediatric drugs nimesulide and PPA – India Medical Times".
- ^ a b Jones G (2008). "Caffeine and other sympathomimetic stimulants: modes of action and effects on sports performance". Essays Biochem. 44: 109–123. doi:10.1042/BSE0440109. PMID 18384286.
- ^ "The Prohibited List". World Anti Doping Agency. 1 January 2024. Retrieved 1 August 2024.
- ^ "Rinexin in Farmaceutiska Specialiteter i Sverige" ["Rinexin" from the Pharmaceutical Specialties of Sweden] (drug catalog) (in Swedish). Retrieved 7 January 2014.
- ^ "Phenylpopanolamine Advisory" (Press release). US Food and Drug Administration. 6 November 2000. Archived from the original on 26 January 2010.
- ^ "Phenylpropanolamine (PPA) Information Page – FDA moves PPA from OTC" (Press release). US Food and Drug Administration. 23 December 2005. Archived from the original on 12 January 2009.
- ^ Over-the-Counter (OTC) Drug Review | OTC Monograph Reform in the CARES Act
- ^ Implementation of the international drug control treaties: changes in the scope of control of substances. Vienna: Commission on Narcotic Drugs, Forty-third session. 6–15 March 2000. Archived from the original on 14 August 2003.
- ^ "Unsafe Drugs- nimesulide, Cisapride, Phenylpropanolamine Banned". 27 January 2011.
- ^ "Madras High Court Revokes Ban on Manufacture and Sale PPA". Scribd.com. 13 September 2011. Retrieved 7 January 2014.
- ^ Gupta RC (23 April 2012). Veterinary Toxicology: Basic and Clinical Principles. Academic Press. pp. 458–. ISBN 978-0-12-385927-3.
- ^ Riviere JE, Papich MG (17 March 2009). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. pp. 1309–. ISBN 978-0-8138-2061-3.
- Amphetamine alkaloids
- Anorectics
- Anti-obesity drugs
- Antihypotensive agents
- Beta-Hydroxyamphetamines
- Decongestants
- Drugs acting on the cardiovascular system
- Drugs acting on the nervous system
- Drugs in sport
- Enantiopure drugs
- Ergogenic aids
- Human drug metabolites
- Norepinephrine releasing agents
- Peripherally selective drugs
- Recreational drug metabolites
- Stimulants
- Sympathomimetics
- Veterinary drugs
- Withdrawn anti-obesity drugs
- World Anti-Doping Agency prohibited substances