MAX (gene)
MAX (also known as myc-associated factor X) is a gene that in humans encodes the MAX transcription factor.[5][6]
Function
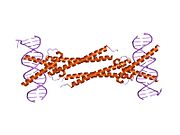
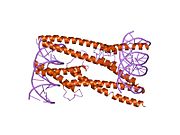
[edit]The protein product of MAX contains the basic helix-loop-helix and leucine zipper motifs. It is therefore included in the bHLHZ family of transcription factors. It is able to form homodimers with other MAX proteins and heterodimers with other transcription factors, including Mad, Mxl1 and Myc. The homodimers and heterodimers compete for a common DNA target site (the E-box) in a gene promoter zone. Rearrangement of dimers (e.g., Mad:Max, Max:Myc) provides a system of transcriptional regulation with greater diversity of gene targets. Max must dimerise in order to be biologically active.[7]
Transcriptionally active hetero- and homodimers involving Max can promote cell proliferation as well as apoptosis.[8]
Interactions
[edit]The protein product of Max has been shown to interact with:
- Myc,[9][10][11][12][13][14][15][16][17][18][19][20][21]
- MNT,[18]
- MSH2,[13]
- MXD1,[15][17][19][22]
- MXI1,[16][18][20][23]
- MYCL1,[14][20]
- N-Myc,[14][20]
- SPAG9,[15]
- TEAD1,[24] and
- Transformation/transcription domain-associated protein.[10][11]
Clinical relevance
[edit]This gene has been shown mutated in cases of hereditary pheochromocytoma.[25] More recently the Max gene becomes mutated and becomes inactivated in small cell lung cancer (SCLC). This is mutually exclusive with alterations at Myc and BRG1, the latter coding for an ATPase of the SWI/SNF complex. It was demonstrated that the BRG1 product regulates the expression of Max through direct recruitment to the Max promoter region, and that depletion of BRG1 strongly hinders cell growth, specifically in Max-deficient cells, suggesting that the two together cause synthetic lethality. Furthermore, Max required BRG1 to activate neuroendocrine transcriptional programs and to up-regulate Myc targets, such as glycolytic-related genes.[26]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000125952 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000059436 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Wagner AJ, Le Beau MM, Diaz MO, Hay N (April 1992). "Expression, regulation, and chromosomal localization of the Max gene". Proceedings of the National Academy of Sciences of the United States of America. 89 (7): 3111–3115. Bibcode:1992PNAS...89.3111W. doi:10.1073/pnas.89.7.3111. PMC 48814. PMID 1557420.
- ^ "Entrez Gene: MAX MYC associated factor X".
- ^ Ecevit O, Khan MA, Goss DJ (March 2010). "Kinetic analysis of the interaction of b/HLH/Z transcription factors Myc, Max, and Mad with cognate DNA". Biochemistry. 49 (12): 2627–2635. doi:10.1021/bi901913a. PMC 2852888. PMID 20170194.
- ^ Amati B, Land H (February 1994). "Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death". Current Opinion in Genetics & Development. 4 (1): 102–108. doi:10.1016/0959-437X(94)90098-1. PMID 8193530.
- ^ Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3: 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- ^ a b McMahon SB, Wood MA, Cole MD (January 2000). "The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc". Molecular and Cellular Biology. 20 (2): 556–562. doi:10.1128/MCB.20.2.556-562.2000. PMC 85131. PMID 10611234.
- ^ a b McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD (August 1998). "The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins". Cell. 94 (3): 363–374. doi:10.1016/S0092-8674(00)81479-8. PMID 9708738. S2CID 17693834.
- ^ Cheng SW, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV (May 1999). "c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function". Nature Genetics. 22 (1): 102–105. doi:10.1038/8811. PMID 10319872. S2CID 12945791.
- ^ a b Mac Partlin M, Homer E, Robinson H, McCormick CJ, Crouch DH, Durant ST, et al. (February 2003). "Interactions of the DNA mismatch repair proteins MLH1 and MSH2 with c-MYC and MAX". Oncogene. 22 (6): 819–825. doi:10.1038/sj.onc.1206252. PMID 12584560.
- ^ a b c Blackwood EM, Eisenman RN (March 1991). "Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc". Science. 251 (4998): 1211–1217. Bibcode:1991Sci...251.1211B. doi:10.1126/science.2006410. PMID 2006410.
- ^ a b c Lee CM, Onésime D, Reddy CD, Dhanasekaran N, Reddy EP (October 2002). "JLP: A scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors". Proceedings of the National Academy of Sciences of the United States of America. 99 (22): 14189–14194. Bibcode:2002PNAS...9914189L. doi:10.1073/pnas.232310199. PMC 137859. PMID 12391307.
- ^ a b Billin AN, Eilers AL, Queva C, Ayer DE (December 1999). "Mlx, a novel Max-like BHLHZip protein that interacts with the Max network of transcription factors". The Journal of Biological Chemistry. 274 (51): 36344–36350. doi:10.1074/jbc.274.51.36344. PMID 10593926.
- ^ a b Gupta K, Anand G, Yin X, Grove L, Prochownik EV (March 1998). "Mmip1: a novel leucine zipper protein that reverses the suppressive effects of Mad family members on c-myc". Oncogene. 16 (9): 1149–1159. doi:10.1038/sj.onc.1201634. PMID 9528857.
- ^ a b c Meroni G, Reymond A, Alcalay M, Borsani G, Tanigami A, Tonlorenzi R, et al. (May 1997). "Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor". The EMBO Journal. 16 (10): 2892–2906. doi:10.1093/emboj/16.10.2892. PMC 1169897. PMID 9184233.
- ^ a b Nair SK, Burley SK (January 2003). "X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors". Cell. 112 (2): 193–205. doi:10.1016/S0092-8674(02)01284-9. PMID 12553908. S2CID 16142388.
- ^ a b c d FitzGerald MJ, Arsura M, Bellas RE, Yang W, Wu M, Chin L, et al. (April 1999). "Differential effects of the widely expressed dMax splice variant of Max on E-box vs initiator element-mediated regulation by c-Myc". Oncogene. 18 (15): 2489–2498. doi:10.1038/sj.onc.1202611. PMID 10229200.
- ^ Meroni G, Cairo S, Merla G, Messali S, Brent R, Ballabio A, et al. (July 2000). "Mlx, a new Max-like bHLHZip family member: the center stage of a novel transcription factors regulatory pathway?". Oncogene. 19 (29): 3266–3277. doi:10.1038/sj.onc.1203634. PMID 10918583.
- ^ Ayer DE, Kretzner L, Eisenman RN (January 1993). "Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity". Cell. 72 (2): 211–222. doi:10.1016/0092-8674(93)90661-9. PMID 8425218. S2CID 13317223.
- ^ Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, et al. (October 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–1178. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- ^ Gupta MP, Amin CS, Gupta M, Hay N, Zak R (July 1997). "Transcription enhancer factor 1 interacts with a basic helix-loop-helix zipper protein, Max, for positive regulation of cardiac alpha-myosin heavy-chain gene expression". Molecular and Cellular Biology. 17 (7): 3924–3936. doi:10.1128/mcb.17.7.3924. PMC 232245. PMID 9199327.
- ^ Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, Landa I, Leandro-García LJ, Letón R, et al. (June 2011). "Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma". Nature Genetics. 43 (7): 663–667. doi:10.1038/ng.861. PMID 21685915. S2CID 205357831.
- ^ Romero OA, Torres-Diz M, Pros E, Savola S, Gomez A, Moran S, et al. (March 2014). "MAX inactivation in small cell lung cancer disrupts MYC-SWI/SNF programs and is synthetic lethal with BRG1". Cancer Discovery. 4 (3): 292–303. doi:10.1158/2159-8290.CD-13-0799. PMID 24362264.
Further reading
[edit]- Grandori C, Cowley SM, James LP, Eisenman RN (2001). "The Myc/Max/Mad network and the transcriptional control of cell behavior". Annual Review of Cell and Developmental Biology. 16: 653–699. doi:10.1146/annurev.cellbio.16.1.653. PMID 11031250.
- Lüscher B (October 2001). "Function and regulation of the transcription factors of the Myc/Max/Mad network". Gene. 277 (1–2): 1–14. doi:10.1016/S0378-1119(01)00697-7. PMID 11602341.
- Wechsler DS, Dang CV (August 1992). "Opposite orientations of DNA bending by c-Myc and Max". Proceedings of the National Academy of Sciences of the United States of America. 89 (16): 7635–7639. Bibcode:1992PNAS...89.7635W. doi:10.1073/pnas.89.16.7635. PMC 49765. PMID 1323849.
- Mäkelä TP, Koskinen PJ, Västrik I, Alitalo K (April 1992). "Alternative forms of Max as enhancers or suppressors of Myc-ras cotransformation". Science. 256 (5055): 373–377. Bibcode:1992Sci...256..373M. doi:10.1126/science.256.5055.373. PMID 1566084. S2CID 37558098.
- Gilladoga AD, Edelhoff S, Blackwood EM, Eisenman RN, Disteche CM (June 1992). "Mapping of MAX to human chromosome 14 and mouse chromosome 12 by in situ hybridization". Oncogene. 7 (6): 1249–1251. PMID 1594250.
- Blackwood EM, Eisenman RN (March 1991). "Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc". Science. 251 (4998): 1211–1217. Bibcode:1991Sci...251.1211B. doi:10.1126/science.2006410. PMID 2006410.
- Zervos AS, Faccio L, Gatto JP, Kyriakis JM, Brent R (November 1995). "Mxi2, a mitogen-activated protein kinase that recognizes and phosphorylates Max protein". Proceedings of the National Academy of Sciences of the United States of America. 92 (23): 10531–10534. Bibcode:1995PNAS...9210531Z. doi:10.1073/pnas.92.23.10531. PMC 40645. PMID 7479834.
- Bousset K, Henriksson M, Lüscher-Firzlaff JM, Litchfield DW, Lüscher B (December 1993). "Identification of casein kinase II phosphorylation sites in Max: effects on DNA-binding kinetics of Max homo- and Myc/Max heterodimers". Oncogene. 8 (12): 3211–3220. PMID 8247525.
- Ayer DE, Kretzner L, Eisenman RN (January 1993). "Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity". Cell. 72 (2): 211–222. doi:10.1016/0092-8674(93)90661-9. PMID 8425218. S2CID 13317223.
- Västrik I, Koskinen PJ, Alitalo R, Mäkelä TP (February 1993). "Alternative mRNA forms and open reading frames of the max gene". Oncogene. 8 (2): 503–507. PMID 8426752.
- Ferré-D'Amaré AR, Prendergast GC, Ziff EB, Burley SK (May 1993). "Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain". Nature. 363 (6424): 38–45. Bibcode:1993Natur.363...38F. doi:10.1038/363038a0. PMID 8479534. S2CID 4304430.
- Hurlin PJ, Quéva C, Koskinen PJ, Steingrímsson E, Ayer DE, Copeland NG, et al. (November 1995). "Mad3 and Mad4: novel Max-interacting transcriptional repressors that suppress c-myc dependent transformation and are expressed during neural and epidermal differentiation". The EMBO Journal. 14 (22): 5646–5659. doi:10.1002/j.1460-2075.1995.tb00252.x. PMC 394680. PMID 8521822.
- Grandori C, Mac J, Siëbelt F, Ayer DE, Eisenman RN (August 1996). "Myc-Max heterodimers activate a DEAD box gene and interact with multiple E box-related sites in vivo". The EMBO Journal. 15 (16): 4344–4357. doi:10.1002/j.1460-2075.1996.tb00808.x. PMC 452159. PMID 8861962.
- Brownlie P, Ceska T, Lamers M, Romier C, Stier G, Teo H, et al. (April 1997). "The crystal structure of an intact human Max-DNA complex: new insights into mechanisms of transcriptional control". Structure. 5 (4): 509–520. doi:10.1016/S0969-2126(97)00207-4. PMID 9115440.
- Meroni G, Reymond A, Alcalay M, Borsani G, Tanigami A, Tonlorenzi R, et al. (May 1997). "Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor". The EMBO Journal. 16 (10): 2892–2906. doi:10.1093/emboj/16.10.2892. PMC 1169897. PMID 9184233.
- Gupta MP, Amin CS, Gupta M, Hay N, Zak R (July 1997). "Transcription enhancer factor 1 interacts with a basic helix-loop-helix zipper protein, Max, for positive regulation of cardiac alpha-myosin heavy-chain gene expression". Molecular and Cellular Biology. 17 (7): 3924–3936. doi:10.1128/mcb.17.7.3924. PMC 232245. PMID 9199327.
- Gupta K, Anand G, Yin X, Grove L, Prochownik EV (March 1998). "Mmip1: a novel leucine zipper protein that reverses the suppressive effects of Mad family members on c-myc". Oncogene. 16 (9): 1149–1159. doi:10.1038/sj.onc.1201634. PMID 9528857.
- Lavigne P, Crump MP, Gagné SM, Hodges RS, Kay CM, Sykes BD (August 1998). "Insights into the mechanism of heterodimerization from the 1H-NMR solution structure of the c-Myc-Max heterodimeric leucine zipper". Journal of Molecular Biology. 281 (1): 165–181. doi:10.1006/jmbi.1998.1914. PMID 9680483.
- FitzGerald MJ, Arsura M, Bellas RE, Yang W, Wu M, Chin L, et al. (April 1999). "Differential effects of the widely expressed dMax splice variant of Max on E-box vs initiator element-mediated regulation by c-Myc". Oncogene. 18 (15): 2489–2498. doi:10.1038/sj.onc.1202611. PMID 10229200.
External links
[edit]- MAX+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- FactorBook Max
This article incorporates text from the United States National Library of Medicine, which is in the public domain.