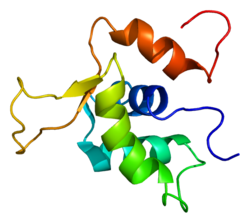
FOXK1
Forkhead box protein K1 is a transcription factor of the forkhead box family that in humans is encoded by the FOXK1 gene.[5][6]
During starvation, in type 2 diabetes, in rapidly dividing cells during embryogenesis, in tumors (Warburg effect) and during T cell proliferation, aerobic glycolysis is induced to produce the building block to sustain growth. FOXK1 is one of the transcription factors managing the passage from the normal cellular respiration (complete glucose oxidation) to generating ATP and intermediaries for many other biochemical pathways.[7]
FOXK1 and its closely relate sibling FOXK2 induce aerobic glycolysis by upregulating the enzymatic machinery required for this (for example, hexokinase-2, phosphofructokinase, pyruvate kinase, and lactate dehydrogenase), while at the same time suppressing further oxidation of pyruvate in the mitochondria by increasing the activity of pyruvate dehydrogenase kinases 1 and 4. Together with suppression of the catalytic subunit of pyruvate dehydrogenase phosphatase 1 this leads to increased phosphorylation of the E1α regulatory subunit of the pyruvate dehydrogenase complex, which in turn inhibits further oxidation of pyruvate in the mitochondria—instead, pyruvate is reduced to lactate. Suppression of FOXK1 and FOXK2 induce the opposite phenotype. Both in vitro and in vivo experiments, including studies of primary human cells, show how FOXK1 and/or FOXK2 are likely to act as important regulators that reprogram cellular metabolism to induce aerobic glycolysis.[7]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000164916 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000056493 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Katoh M, Katoh M (July 2004). "Identification and characterization of human FOXK1 gene in silico". International Journal of Molecular Medicine. 14 (1): 127–132. doi:10.3892/ijmm.14.1.127. PMID 15202027.
- ^ "Entrez Gene: FOXK1 forkhead box K1".
- ^ a b Sukonina V, Ma H, Zhang W, Bartesaghi S, Subhash S, Heglind M, et al. (February 2019). "FOXK1 and FOXK2 regulate aerobic glycolysis". Nature. 566 (7743): 279–283. Bibcode:2019Natur.566..279S. doi:10.1038/s41586-019-0900-5. PMID 30700909. S2CID 59524697.
Further reading
[edit]- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. (November 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–648. doi:10.1016/j.cell.2006.09.026. PMID 17081983. S2CID 7827573.
- Tsai KL, Huang CY, Chang CH, Sun YJ, Chuang WJ, Hsiao CD (June 2006). "Crystal structure of the human FOXK1a-DNA complex and its implications on the diverse binding specificity of winged helix/forkhead proteins". The Journal of Biological Chemistry. 281 (25): 17400–17409. doi:10.1074/jbc.M600478200. PMID 16624804.
- Huang JT, Lee V (September 2004). "Identification and characterization of a novel human FOXK1 gene in silico". International Journal of Oncology. 25 (3): 751–757. doi:10.3892/ijo.25.3.751. PMID 15289879.