Adrenaline
Adrenaline, also known as epinephrine, is a hormone and medication[10][11] which is involved in regulating visceral functions (e.g., respiration).[10][12] It appears as a white microcrystalline granule.[13] Adrenaline is normally produced by the adrenal glands and by a small number of neurons in the medulla oblongata.[14] It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output by acting on the SA node,[15] pupil dilation response, and blood sugar level.[16][17] It does this by binding to alpha and beta receptors.[17] It is found in many animals, including humans, and some single-celled organisms.[18][19] It has also been isolated from the plant Scoparia dulcis found in Northern Vietnam.[20]
Medical uses
[edit]As a medication, it is used to treat several conditions, including allergic reaction anaphylaxis, cardiac arrest, and superficial bleeding.[8] Inhaled adrenaline may be used to improve the symptoms of croup.[21] It may also be used for asthma when other treatments are not effective. It is given intravenously, by injection into a muscle, by inhalation, or by injection just under the skin.[8] Common side effects include shakiness, anxiety, and sweating. A fast heart rate and high blood pressure may occur. Occasionally it may result in an abnormal heart rhythm. While the safety of its use during pregnancy and breastfeeding is unclear, the benefits to the mother must be taken into account.[8]
A case has been made for the use of adrenaline infusion in place of the widely accepted treatment of inotropes for preterm infants with clinical cardiovascular compromise. Although sufficient data strongly recommends adrenaline infusions as a viable treatment, more trials are needed to conclusively determine that these infusions will successfully reduce morbidity and mortality rates among preterm, cardiovascularly compromised infants.[22]
Epinephrine can also be used to treat open-angle glaucoma, as it has been found to increase the outflow of aqueous humor in the eye. This lowers the intraocular pressure in the eye and thus aids in treatment.[23]
Physiological effects
[edit]The adrenal medulla is a major contributor to total circulating catecholamines (L-DOPA is at a higher concentration in the plasma),[24] though it contributes over 90% of circulating adrenaline. Little adrenaline is found in other tissues, mostly in scattered chromaffin cells and in a small number of neurons that use adrenaline as a neurotransmitter.[25] Following adrenalectomy, adrenaline disappears below the detection limit in the bloodstream.[26]
Pharmacological doses of adrenaline stimulate α1, α2, β1, β2, and β3 adrenoceptors of the sympathetic nervous system. Sympathetic nerve receptors are classified as adrenergic, based on their responsiveness to adrenaline.[27] The term "adrenergic" is often misinterpreted in that the main sympathetic neurotransmitter is noradrenaline, rather than adrenaline, as discovered by Ulf von Euler in 1946.[28][29] Adrenaline has a β2 adrenoceptor-mediated effect on metabolism and the airway, with no direct neural connection from the sympathetic ganglia to the airway.[30][31][32]
Walter Bradford Cannon originally proposed the concept of the adrenal medulla and the sympathetic nervous system being involved in the flight, fight, and fright response.[33] But the adrenal medulla, in contrast to the adrenal cortex, is not required for survival. In adrenalectomized patients, hemodynamic and metabolic responses to stimuli such as hypoglycemia and exercise remain normal.[34]
Exercise
[edit]One physiological stimulus to adrenaline secretion is exercise. This was first demonstrated by measuring the dilation of a (denervated) pupil of a cat on a treadmill,[35] later confirmed using a biological assay of urine samples.[36] Biochemical methods for measuring catecholamines in plasma were published from 1950 onwards.[37] Although much valuable work has been published using fluorimetric assays to measure total catecholamine concentrations, the method is too non-specific and insensitive to accurately determine the very small quantities of adrenaline in plasma. The development of extraction methods and enzyme–isotope derivate radio-enzymatic assays (REA) transformed the analysis down to a sensitivity of 1 pg for adrenaline.[38] Early REA plasma assays indicated that adrenaline and total catecholamines rise late in exercise, mostly when anaerobic metabolism commences.[39][40][41]
During exercise, the adrenaline blood concentration rises partially from the increased secretion of the adrenal medulla and partly from the decreased metabolism of adrenaline due to reduced blood flow to the liver.[42] Infusion of adrenaline to reproduce exercise circulating concentrations of adrenaline in subjects at rest has little hemodynamic effect other than a slight β2-mediated fall in diastolic blood pressure.[43][44] Infusion of adrenaline well within the physiological range suppresses human airway hyper-reactivity sufficiently to antagonize the constrictor effects of inhaled histamine.[45]
A link between the sympathetic nervous system and the lungs was shown in 1887 when Grossman showed that stimulation of cardiac accelerator nerves reversed muscarine-induced airway constriction.[46] In experiments in the dog, where the sympathetic chain was cut at the level of the diaphragm, Jackson showed that there was no direct sympathetic innervation to the lung, but bronchoconstriction was reversed by the release of adrenaline from the adrenal medulla.[47] An increased incidence of asthma has not been reported for adrenalectomized patients; those with a predisposition to asthma will have some protection from airway hyper-reactivity from their corticosteroid replacement therapy. Exercise induces progressive airway dilation in normal subjects that correlates with workload and is not prevented by beta-blockade.[48] The progressive airway dilation with increasing exercise is mediated by a progressive reduction in resting vagal tone. Beta blockade with propranolol causes a rebound in airway resistance after exercise in normal subjects over the same time course as the bronchoconstriction seen with exercise-induced asthma.[49] The reduction in airway resistance during exercise reduces the work of breathing.[50]
Emotional responses
[edit]Every emotional response has a behavioral, an autonomic, and a hormonal component. The hormonal component includes the release of adrenaline, an adrenomedullary response to stress controlled by the sympathetic nervous system. The major emotion studied in relation to adrenaline is fear. In an experiment, subjects who were injected with adrenaline expressed more negative and fewer positive facial expressions to fear films compared to a control group. These subjects also reported a more intense fear from the films and greater mean intensity of negative memories than control subjects.[51] The findings from this study demonstrate that there are learned associations between negative feelings and levels of adrenaline. Overall, the greater amount of adrenaline is positively correlated with an aroused state of negative emotions. These findings can be an effect in part that adrenaline elicits physiological sympathetic responses, including an increased heart rate and knee shaking, which can be attributed to the feeling of fear regardless of the actual level of fear elicited from the video. Although studies have found a definite relation between adrenaline and fear, other emotions have not had such results. In the same study, subjects did not express a greater amusement to an amusement film nor greater anger to an anger film.[51] Similar findings were also supported in a study that involved rodent subjects that either were able or unable to produce adrenaline. Findings support the idea that adrenaline has a role in facilitating the encoding of emotionally arousing events, contributing to higher levels of arousal due to fear.[52]
Memory
[edit]It has been found that adrenergic hormones, such as adrenaline, can produce retrograde enhancement of long-term memory in humans. The release of adrenaline due to emotionally stressful events, which is endogenous adrenaline, can modulate memory consolidation of the events, ensuring memory strength that is proportional to memory importance. Post-learning adrenaline activity also interacts with the degree of arousal associated with the initial coding.[53] There is evidence that suggests adrenaline does have a role in long-term stress adaptation and emotional memory encoding specifically. Adrenaline may also play a role in elevating arousal and fear memory under particular pathological conditions, including post-traumatic stress disorder.[52] Overall, "Extensive evidence indicates that epinephrine (EPI) modulates memory consolidation for emotionally arousing tasks in animals and human subjects."[54] Studies have also found that recognition memory involving adrenaline depends on a mechanism that depends on β adrenoceptors.[54] Adrenaline does not readily cross the blood-brain barrier, so its effects on memory consolidation are at least partly initiated by β adrenoceptors in the periphery. Studies have found that sotalol, a β adrenoceptor antagonist that also does not readily enter the brain, blocks the enhancing effects of peripherally administered adrenaline on memory.[55] These findings suggest that β adrenoceptors are necessary for adrenaline to have an impact on memory consolidation.[56][57]
Pathology
[edit]Increased adrenaline secretion is observed in pheochromocytoma, hypoglycemia, myocardial infarction, and to a lesser degree, in essential tremor (also known as benign, familial, or idiopathic tremor). A general increase in sympathetic neural activity is usually accompanied by increased adrenaline secretion, but there is selectivity during hypoxia and hypoglycemia, when the ratio of adrenaline to noradrenaline is considerably increased.[58][59][60] Therefore, there must be some autonomy of the adrenal medulla from the rest of the sympathetic system.
Myocardial infarction is associated with high levels of circulating adrenaline and noradrenaline, particularly in cardiogenic shock.[61][62]
Benign familial tremor (essential tremor) (BFT) is responsive to peripheral β adrenergic blockers, and β2-stimulation is known to cause tremor. Patients with BFT were found to have increased plasma adrenaline but not noradrenaline.[63][64]
Low or absent concentrations of adrenaline can be seen in autonomic neuropathy or following adrenalectomy. Failure of the adrenal cortex, as with Addison's disease, can suppress adrenaline secretion as the activity of the synthesizing enzyme, phenylethanolamine-N-methyltransferase, depends on the high concentration of cortisol that drains from the cortex to the medulla.[65][66][67]
Terminology
[edit]In 1901, Jōkichi Takamine patented a purified extract from the adrenal glands, which was trademarked by Parke, Davis & Co in the US.[68] The British Approved Name and European Pharmacopoeia term for this drug is hence adrenaline (from Latin ad, "on", and rēnālis, "of the kidney", from ren, "kidney").[69]
However, the pharmacologist John Abel had already prepared an extract from adrenal glands as early as 1897, and he coined the name epinephrine to describe it (from Ancient Greek ἐπῐ́ (epí), "upon", and νεφρός (nephrós), "kidney").[68] As the term Adrenaline was a registered trademark in the US,[68] and in the belief that Abel's extract was the same as Takamine's (a belief since disputed), epinephrine instead became[when?] the generic name used in the US[68] and remains the pharmaceutical's United States Adopted Name and International Nonproprietary Name (though the name adrenaline is frequently used[70]).
The terminology is now one of the few differences between the INN and BAN systems of names.[71] Although European health professionals and scientists preferentially use the term adrenaline, the converse is true among American health professionals and scientists. Nevertheless, even among the latter, receptors for this substance are called adrenergic receptors or adrenoceptors, and pharmaceuticals that mimic its effects are often called adrenergics. The history of adrenaline and epinephrine is reviewed by Rao.[72]
Mechanism of action
[edit]| Organ | Effects |
|---|---|
| Heart | Increases heart rate; contractility; conduction across AV node |
| Lungs | Increases respiratory rate; bronchodilation |
| Liver | Stimulates glycogenolysis |
| Muscle | Stimulates glycogenolysis and glycolysis |
| Brain | Increased cerebral tissue oxygenation |
| Systemic | Vasoconstriction and vasodilation |
| Triggers lipolysis | |
| Muscle contraction |
As a hormone, adrenaline acts on nearly all body tissues by binding to adrenergic receptors. Its effects on various tissues depend on the type of tissue and expression of specific forms of adrenergic receptors. For example, high levels of adrenaline cause smooth muscle relaxation in the airways but causes contraction of the smooth muscle that lines most arterioles.
Adrenaline is a nonselective agonist of all adrenergic receptors, including the major subtypes α1, α2, β1, β2, and β3.[73] Adrenaline's binding to these receptors triggers a number of metabolic changes. Binding to α-adrenergic receptors inhibits insulin secretion by the pancreas, stimulates glycogenolysis in the liver and muscle,[74] and stimulates glycolysis and inhibits insulin-mediated glycogenesis in muscle.[75][76] β adrenergic receptor binding triggers glucagon secretion in the pancreas, increased adrenocorticotropic hormone (ACTH) secretion by the pituitary gland, and increased lipolysis by adipose tissue. Together, these effects increase blood glucose and fatty acids, providing substrates for energy production within cells throughout the body.[76] Binding of β adrenergic receptor also increases the production of cyclic AMP.[77]
Adrenaline causes liver cells to release glucose into the blood, acting through both alpha and beta-adrenergic receptors to stimulate glycogenolysis. Adrenaline binds to β2 receptors on liver cells, which changes conformation and helps Gs, a heterotrimeric G protein, exchange GDP to GTP. This trimeric G protein dissociates to Gs alpha and Gs beta/gamma subunits. Gs alpha stimulates adenylyl cyclase, thus converting adenosine triphosphate into cyclic adenosine monophosphate (AMP). Cyclic AMP activates protein kinase A. Protein kinase A phosphorylates and partially activates phosphorylase kinase. Adrenaline also binds to α1 adrenergic receptors, causing an increase in inositol trisphosphate, inducing calcium ions to enter the cytoplasm. Calcium ions bind to calmodulin, which leads to further activation of phosphorylase kinase. Phosphorylase kinase phosphorylates glycogen phosphorylase, which then breaks down glycogen leading to the production of glucose.[78]
Adrenaline also has significant effects on the cardiovascular system. It increases peripheral resistance via α1 receptor-dependent vasoconstriction and increases cardiac output by binding to β1 receptors. The goal of reducing peripheral circulation is to increase coronary and cerebral perfusion pressures and therefore increase oxygen exchange at the cellular level.[79][80] While adrenaline does increase aortic, cerebral, and carotid circulation pressure, it lowers carotid blood flow and end-tidal CO2 or ETCO2 levels. It appears that adrenaline improves microcirculation at the expense of the capillary beds where perfusion takes place.[81]
Measurement in biological fluids
[edit]Adrenaline may be quantified in blood, plasma, or serum as a diagnostic aid, to monitor therapeutic administration, or to identify the causative agent in a potential poisoning victim. Endogenous plasma adrenaline concentrations in resting adults usually are less than 10 ng/L, but they may increase by 10-fold during exercise and by 50-fold or more during times of stress. Pheochromocytoma patients often have plasma adrenaline levels of 1000–10,000 ng/L. Parenteral administration of adrenaline to acute-care cardiac patients can produce plasma concentrations of 10,000 to 100,000 ng/L.[82][83]
Biosynthesis
[edit]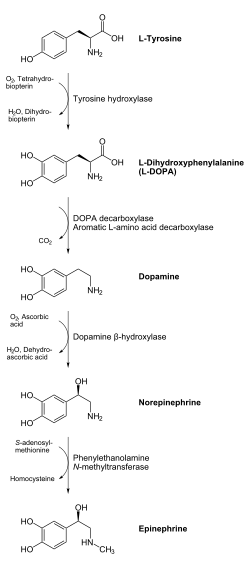
In chemical terms, adrenaline is one of a group of monoamines called the catecholamines. Adrenaline is synthesized in the chromaffin cells of the adrenal gland's adrenal medulla and a small number of neurons in the medulla oblongata in the brain through a metabolic pathway that converts the amino acids phenylalanine and tyrosine into a series of metabolic intermediates and, ultimately, adrenaline.[10][12][84] Tyrosine is first oxidized to L-DOPA by tyrosine hydroxylase; this is the rate-limiting step. Then it is subsequently decarboxylated to give dopamine by DOPA decarboxylase (aromatic L-amino acid decarboxylase). Dopamine is then converted to noradrenaline by dopamine beta-hydroxylase, which utilizes ascorbic acid (vitamin C) and copper. The final step in adrenaline biosynthesis is the methylation of the primary amine of noradrenaline. This reaction is catalyzed by the enzyme phenylethanolamine N-methyltransferase (PNMT), which utilizes S-adenosyl methionine (SAMe) as the methyl donor.[85] While PNMT is found primarily in the cytosol of the endocrine cells of the adrenal medulla (also known as chromaffin cells), it has been detected at low levels in both the heart and brain.[86]
Regulation
[edit]The major physiologic triggers of adrenaline release center upon stresses, such as physical threat, excitement, noise, bright lights, and high or low ambient temperature. All of these stimuli are processed in the central nervous system.[90]
Adrenocorticotropic hormone (ACTH) and the sympathetic nervous system stimulate the synthesis of adrenaline precursors by enhancing the activity of tyrosine hydroxylase and dopamine β-hydroxylase, two key enzymes involved in catecholamine synthesis.[citation needed] ACTH also stimulates the adrenal cortex to release cortisol, which increases the expression of PNMT in chromaffin cells, enhancing adrenaline synthesis. This is most often done in response to stress.[citation needed] The sympathetic nervous system, acting via splanchnic nerves to the adrenal medulla, stimulates the release of adrenaline. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and, thus, the release of adrenaline (and noradrenaline) into the bloodstream.[citation needed] For noradrenaline to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H+ exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized adrenaline from the cytosol back into chromaffin granules in preparation for release.[91]
Unlike many other hormones, adrenaline (as with other catecholamines) does not exert negative feedback to down-regulate its own synthesis. Abnormal adrenaline levels can occur in various conditions, such as surreptitious adrenaline administration, pheochromocytoma, and other tumors of the sympathetic ganglia.
Its action is terminated with reuptake into nerve terminal endings, some minute dilution, and metabolism by monoamine oxidase[92] and catechol-O-methyl transferase into 3,4-Dihydroxymandelic acid and Metanephrine.
History
[edit]Extracts of the adrenal gland were first obtained by Polish physiologist Napoleon Cybulski in 1895.[93] These extracts, which he called nadnerczyna ("adrenalin"), contained adrenaline and other catecholamines.[94] American ophthalmologist William H. Bates discovered adrenaline's usage for eye surgeries prior to 20 April 1896.[95] In 1897, John Jacob Abel (1857–1938), the father of modern pharmacology, found a natural substance produced by the adrenal glands that he named epinephrine. The first hormone to be identified, it remains a crucial, first-line treatment for cardiac arrests, severe allergic reactions, and other conditions. In 1901, Jokichi Takamine successfully isolated and purified the hormone from the adrenal glands of sheep and oxen.[96] Adrenaline was first synthesized in the laboratory by Friedrich Stolz and Henry Drysdale Dakin, independently, in 1904.[97]
Although secretin is mentioned as the first hormone, adrenaline is the first hormone since the discovery of the activity of adrenal extract on blood pressure was observed in 1895 before that of secretin in 1902.[72] In 1895, George Oliver (1841–1915), a general practitioner in North Yorkshire, and Edward Albert Schäfer (1850–1935), a physiologist at University College of London published a paper about the active component of adrenal gland extract causing the increase in blood pressure and heart rate was from the medulla, but not the cortex of the adrenal gland.[98] In 1897, John Jacob Abel (1857–1938) of Johns Hopkins University, the first chairman of the first US department of pharmacology, found a compound called epinephrine with the molecular formula of C17H15NO4.[72] Abel claimed his principle from adrenal gland extract was active.
In 1900, Jōkichi Takamine (1854–1922), a Japanese chemist, worked with his assistant, Keizo Uenaka (1876–1960), to purify a 2000 times more active principle than epinephrine from the adrenal gland, named adrenaline with the molecular formula C10H15NO3.[72][98] Additionally, in 1900 Thomas Aldrich of Parke-Davis Scientific Laboratory also purified adrenaline independently. Takamine and Parke-Davis later in 1901 both got the patent for adrenaline. The fight for terminology between adrenaline and epinephrine was not ended until the first adrenaline structural discovery by Hermann Pauly (1870–1950) in 1903 and the first adrenaline synthesis by Friedrich Stolz (1860–1936), a German chemist in 1904. They both believed that Takamine's compound was the active principle while Abel's compound was the inactive one.[citation needed] Stolz synthesized adrenaline from its ketone form (adrenalone).[99]
Society and culture
[edit]Adrenaline junkie
[edit]An adrenaline junkie is someone who engages in sensation-seeking behavior through "the pursuit of novel and intense experiences without regard for physical, social, legal or financial risk".[100] Such activities include extreme and risky sports, substance abuse, unsafe sex, and crime. The term relates to the increase in circulating levels of adrenaline during physiological stress.[101] Such an increase in the circulating concentration of adrenaline is secondary to the activation of the sympathetic nerves innervating the adrenal medulla, as it is rapid and not present in animals where the adrenal gland has been removed.[102] Although such stress triggers adrenaline release, it also activates many other responses within the central nervous system reward system, which drives behavioral responses; while the circulating adrenaline concentration is present, it may not drive behavior. Nevertheless, adrenaline infusion alone does increase alertness[103] and has roles in the brain, including the augmentation of memory consolidation.[101]
Strength
[edit]Adrenaline has been implicated in feats of great strength, often occurring in times of crisis. For example, there are stories of a parent lifting part of a car when their child is trapped underneath, showcasing the ability of the body to endure under stress and highlighting the significant effects of adrenaline in unlocking extraordinary physical abilities.[104][105]
See also
[edit]References
[edit]- ^ Andersen AM (1975). "Structural Studies of Metabolic Products of Dopamine. III. Crystal and Molecular Structure of (−)-Adrenaline". Acta Chem. Scand. 29b (2): 239–244. doi:10.3891/acta.chem.scand.29b-0239. PMID 1136652.
- ^ "Neffy- epinephrine spray". DailyMed. 20 August 2024. Retrieved 5 September 2024.
- ^ "European Medicines Agency". Eurneffy EPAR. 27 June 2024. Archived from the original on 29 June 2024. Retrieved 29 June 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Eurneffy Product information". Union Register of medicinal products. 23 August 2024. Retrieved 27 August 2024.
- ^ El-Bahr SM, Kahlbacher H, Patzl M, Palme RG (May 2006). "Binding and clearance of radioactive adrenaline and noradrenaline in sheep blood". Veterinary Research Communications. 30 (4). Springer Science and Business Media LLC: 423–32. doi:10.1007/s11259-006-3244-1. PMID 16502110. S2CID 9054777.
- ^ Franksson G, Anggård E (March 2009). "The plasma protein binding of amphetamine, catecholamines and related compounds". Acta Pharmacologica et Toxicologica. 28 (3). Wiley: 209–14. doi:10.1111/j.1600-0773.1970.tb00546.x. PMID 5468075.
- ^ Peaston RT, Weinkove C (January 2004). "Measurement of catecholamines and their metabolites". Annals of Clinical Biochemistry. 41 (Pt 1). SAGE Publications: 17–38. doi:10.1258/000456304322664663. PMID 14713382. S2CID 2330329.
- ^ a b c d "Epinephrine". The American Society of Health-System Pharmacists. Retrieved 15 August 2015.
- ^ Hummel MD (2012). "Emergency Medications". In Pollak AN (ed.). Nancy Caroline's Emergency Care in the Streets (7th ed.). Burlington: Jones & Bartlett Learning. p. 557. ISBN 9781449645861. Archived from the original on 8 September 2017.
- ^ a b c Lieberman M, Marks A, Peet A (2013). Marks' Basic Medical Biochemistry: A Clinical Approach (4th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 175. ISBN 9781608315727.
- ^ "Adrenaline". 21 August 2015.
- ^ a b c Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 157. ISBN 9780071481274.
Epinephrine occurs in only a small number of central neurons, all located in the medulla. Epinephrine is involved in visceral functions, such as the control of respiration. It is also produced by the adrenal medulla.
- ^ Larrañaga M (2016). Hawley's Condensed Chemical Dictionary. New Jersey: John Wiley & Sons, Incorporated. p. 561.
- ^ "Adrenaline: physiology and pharmacology | DermNet". dermnetnz.org. Retrieved 20 March 2023.
- ^ Brown HF, DiFrancesco D, Noble SJ (July 1979). "How does adrenaline accelerate the heart?". Nature. 280 (5719): 235–236. Bibcode:1979Natur.280..235B. doi:10.1038/280235a0. PMID 450140. S2CID 4350616.
- ^ Bell DR (2009). Medical physiology : principles for clinical medicine (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 312. ISBN 9780781768528.
- ^ a b Khurana I (2008). Essentials of Medical Physiology. Elsevier India. p. 460. ISBN 9788131215661.
- ^ Buckley E (2013). Venomous Animals and Their Venoms: Venomous Vertebrates. Elsevier. p. 478. ISBN 9781483262888.
- ^ Animal Physiology: Adaptation and Environment (5th ed.). Cambridge University Press. 1997. p. 510. ISBN 9781107268500.
- ^ Phan MG, Phan TS, Matsunami K, Otsuka H (April 2006). "Chemical and biological evaluation on scopadulane-type diterpenoids from Scoparia dulcis of Vietnamese origin". Chemical & Pharmaceutical Bulletin. 54 (4): 546–549. doi:10.1248/cpb.54.546. PMID 16595962.
- ^ Everard ML (February 2009). "Acute bronchiolitis and croup". Pediatric Clinics of North America. 56 (1): 119–133, x–xi. doi:10.1016/j.pcl.2008.10.007. PMID 19135584.
- ^ Paradisis M, Osborn DA (2004). "Adrenaline for prevention of morbidity and mortality in preterm infants with cardiovascular compromise". The Cochrane Database of Systematic Reviews (1): CD003958. doi:10.1002/14651858.CD003958.pub2. PMID 14974048.
- ^ Erickson-Lamy KA, Nathanson JA (August 1992). "Epinephrine increases facility of outflow and cyclic AMP content in the human eye in vitro". Investigative Ophthalmology & Visual Science. 33 (9): 2672–2678. PMID 1353486.
- ^ Rizzo V, Memmi M, Moratti R, Melzi d'Eril G, Perucca E (June 1996). "Concentrations of L-dopa in plasma and plasma ultrafiltrates". Journal of Pharmaceutical and Biomedical Analysis. 14 (8–10): 1043–1046. doi:10.1016/s0731-7085(96)01753-0. PMID 8818013.
- ^ Fuller RW (April 1982). "Pharmacology of brain epinephrine neurons". Annual Review of Pharmacology and Toxicology. 22 (1): 31–55. doi:10.1146/annurev.pa.22.040182.000335. PMID 6805416.
- ^ Cryer PE (August 1980). "Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system". The New England Journal of Medicine. 303 (8): 436–444. doi:10.1056/nejm198008213030806. PMID 6248784.
- ^ Barger G, Dale HH (October 1910). "Chemical structure and sympathomimetic action of amines". The Journal of Physiology. 41 (1–2): 19–59. doi:10.1113/jphysiol.1910.sp001392. PMC 1513032. PMID 16993040.
- ^ Von Euler US (1946). "A specific sympathomimetic ergone in adrenergic nerve fibres (sympathin) and its relations to adrenaline and nor adrenaline". Acta Physiologica Scandinavica. 12: 73–97. doi:10.1111/j.1748-1716.1946.tb00368.x.
- ^ Von Euler US, Hillarp NA (January 1956). "Evidence for the presence of noradrenaline in submicroscopic structures of adrenergic axons". Nature. 177 (4497): 44–45. Bibcode:1956Natur.177...44E. doi:10.1038/177044b0. PMID 13288591. S2CID 4214745.
- ^ Warren J (January 1986). "The adrenal medulla and the airway". British Journal of Diseases of the Chest. 80 (1): 1–6. doi:10.1016/0007-0971(86)90002-1. PMID 3004549.
- ^ Twentyman OP, Disley A, Gribbin HR, Alberti KG, Tattersfield AE (October 1981). "Effect of beta-adrenergic blockade on respiratory and metabolic responses to exercise". Journal of Applied Physiology. 51 (4): 788–793. doi:10.1152/jappl.1981.51.4.788. PMID 6795164.
- ^ Richter EA, Galbo H, Christensen NJ (January 1981). "Control of exercise-induced muscular glycogenolysis by adrenal medullary hormones in rats". Journal of Applied Physiology. 50 (1). American Physiological Society: 21–26. doi:10.1152/jappl.1981.50.1.21. PMID 7009527.
- ^ Canon WB (1931). "Studies on the conditions of activity in endocrine organs xxvii. Evidence that medulliadrenal secretion is not continuous". The American Journal of Physiology. 98: 447–453. doi:10.1152/ajplegacy.1931.98.3.447.
- ^ Cryer PE, Tse TF, Clutter WE, Shah SD (August 1984). "Roles of glucagon and epinephrine in hypoglycemic and nonhypoglycemic glucose counterregulation in humans". The American Journal of Physiology. 247 (2 Pt 1): E198–E205. doi:10.1152/ajpendo.1984.247.2.E198. PMID 6147094.
- ^ Hartman FA, Waite RH, McCordock HA (1922). "The liberation of epinephrine during muscular exercise". The American Journal of Physiology. 62 (2): 225–241. doi:10.1152/ajplegacy.1922.62.2.225.
- ^ Von Euler US, Hellner S (September 1952). "Excretion of noradrenaline and adrenaline in muscular work". Acta Physiologica Scandinavica. 26 (2–3): 183–191. doi:10.1111/j.1748-1716.1952.tb00900.x. PMID 12985406.
- ^ Lund A (1950). "Simultaneous fluorimetric determinations of adrenaline and noradrenaline in blood". Acta Pharmacologica et Toxicologica. 6 (2): 137–146. doi:10.1111/j.1600-0773.1950.tb03460.x. PMID 24537959.
- ^ Johnson GA, Kupiecki RM, Baker CA (November 1980). "Single isotope derivative (radioenzymatic) methods in the measurement of catecholamines". Metabolism. 29 (11 Suppl 1): 1106–1113. doi:10.1016/0026-0495(80)90018-9. PMID 7001177.
- ^ Galbo H, Holst JJ, Christensen NJ (January 1975). "Glucagon and plasma catecholamine responses to graded and prolonged exercise in man". Journal of Applied Physiology. 38 (1): 70–76. doi:10.1152/jappl.1975.38.1.70. PMID 1110246.
- ^ Winder WW, Hagberg JM, Hickson RC, Ehsani AA, McLane JA (September 1978). "Time course of sympathoadrenal adaptation to endurance exercise training in man". Journal of Applied Physiology. 45 (3): 370–374. doi:10.1152/jappl.1978.45.3.370. PMID 701121.
- ^ Kindermann W, Schnabel A, Schmitt WM, Biro G, Hippchen M (May 1982). "[Catecholamines, GH, cortisol, glucagon, insulin, and sex hormones in exercise and beta 1-blockade (author's transl)]". Klinische Wochenschrift. 60 (10): 505–512. doi:10.1007/bf01756096. PMID 6124653. S2CID 30270788.
- ^ Warren JB, Dalton N, Turner C, Clark TJ, Toseland PA (January 1984). "Adrenaline secretion during exercise". Clinical Science. 66 (1): 87–90. doi:10.1042/cs0660087. PMID 6690194.
- ^ Fitzgerald GA, Barnes P, Hamilton CA, Dollery CT (October 1980). "Circulating adrenaline and blood pressure: the metabolic effects and kinetics of infused adrenaline in man". European Journal of Clinical Investigation. 10 (5): 401–406. doi:10.1111/j.1365-2362.1980.tb00052.x. PMID 6777175. S2CID 38894042.
- ^ Warren JB, Dalton N (May 1983). "A comparison of the bronchodilator and vasopressor effects of exercise levels of adrenaline in man". Clinical Science. 64 (5): 475–479. doi:10.1042/cs0640475. PMID 6831836.
- ^ Warren JB, Dalton N, Turner C, Clark TJ (November 1984). "Protective effect of circulating epinephrine within the physiologic range on the airway response to inhaled histamine in nonasthmatic subjects". The Journal of Allergy and Clinical Immunology. 74 (5): 683–686. doi:10.1016/0091-6749(84)90230-6. PMID 6389647.
- ^ Grossman M (1887). "Das muscarin-lungen-odem". Zeitschrift für klinische Medizin. 12: 550–591.
- ^ Jackson DE (1912). "The pulmonary action of the adrenal glands". Journal of Pharmacology and Experimental Therapeutics. 4: 59–74.
- ^ Kagawa J, Kerr HD (February 1970). "Effects of brief graded exercise on specific airway conductance in normal subjects". Journal of Applied Physiology. 28 (2): 138–144. doi:10.1152/jappl.1970.28.2.138. PMID 5413299.
- ^ Warren JB, Jennings SJ, Clark TJ (January 1984). "Effect of adrenergic and vagal blockade on the normal human airway response to exercise". Clinical Science. 66 (1): 79–85. doi:10.1042/cs0660079. PMID 6228370.
- ^ Jennings SJ, Warren JB, Pride NB (July 1987). "Airway caliber and the work of breathing in humans". Journal of Applied Physiology. 63 (1): 20–24. doi:10.1152/jappl.1987.63.1.20. PMID 2957350.
- ^ a b Mezzacappa ES, Katkin ES, Palmer SN (1999). "Epinephrine, arousal, and emotion: A new look at two-factor theory". Cognition and Emotion. 13 (2): 181–199. doi:10.1080/026999399379320.
- ^ a b Toth M, Ziegler M, Sun P, Gresack J, Risbrough V (February 2013). "Impaired conditioned fear response and startle reactivity in epinephrine-deficient mice". Behavioural Pharmacology. 24 (1): 1–9. doi:10.1097/FBP.0b013e32835cf408. PMC 3558035. PMID 23268986.
- ^ Cahill L, Alkire MT (March 2003). "Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding". Neurobiology of Learning and Memory. 79 (2): 194–198. doi:10.1016/S1074-7427(02)00036-9. PMID 12591227. S2CID 12099979.
- ^ a b Dornelles A, de Lima MN, Grazziotin M, Presti-Torres J, Garcia VA, Scalco FS, et al. (July 2007). "Adrenergic enhancement of consolidation of object recognition memory". Neurobiology of Learning and Memory. 88 (1): 137–142. doi:10.1016/j.nlm.2007.01.005. PMID 17368053. S2CID 27697668.
- ^ Roozendaal B, McGaugh JL (December 2011). "Memory modulation". Behavioral Neuroscience. 125 (6): 797–824. doi:10.1037/a0026187. PMC 3236701. PMID 22122145.
- ^ Tully K, Bolshakov VY (May 2010). "Emotional enhancement of memory: how norepinephrine enables synaptic plasticity". Molecular Brain. 3 (1): 15. doi:10.1186/1756-6606-3-15. PMC 2877027. PMID 20465834.
- ^ Ferry B, Roozendaal B, McGaugh JL (June 1999). "Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors". The Journal of Neuroscience. 19 (12): 5119–5123. doi:10.1523/JNEUROSCI.19-12-05119.1999. PMC 6782651. PMID 10366644.
- ^ Feldberg W, Minz B, Tsudzimura H (June 1934). "The mechanism of the nervous discharge of adrenaline". The Journal of Physiology. 81 (3): 286–304. doi:10.1113/jphysiol.1934.sp003136. PMC 1394156. PMID 16994544.
- ^ Burn JH, Hutcheon DE, Parker RH (September 1950). "Adrenaline and noradrenaline in the suprarenal medulla after insulin". British Journal of Pharmacology and Chemotherapy. 5 (3): 417–423. doi:10.1111/j.1476-5381.1950.tb00591.x. PMC 1509946. PMID 14777865.
- ^ Outschoorn AS (December 1952). "The hormones of the adrenal medulla and their release". British Journal of Pharmacology and Chemotherapy. 7 (4): 605–615. doi:10.1111/j.1476-5381.1952.tb00728.x. PMC 1509311. PMID 13019029.
- ^ Benedict CR, Grahame-Smith DG (August 1979). "Plasma adrenaline and noradrenaline concentrations and dopamine-beta-hydroxylase activity in myocardial infarction with and without cardiogenic shock". British Heart Journal. 42 (2): 214–220. doi:10.1136/hrt.42.2.214. PMC 482137. PMID 486283.
- ^ Nadeau RA, de Champlain J (November 1979). "Plasma catecholamines in acute myocardial infarction". American Heart Journal. 98 (5): 548–554. doi:10.1016/0002-8703(79)90278-3. PMID 495400.
- ^ Larsson S, Svedmyr N (1977). "Tremor caused by sympathomimetics is mediated by beta 2-adrenoceptors". Scandinavian Journal of Respiratory Diseases. 58 (1): 5–10. PMID 190674.
- ^ Warren JB, O'Brien M, Dalton N, Turner CT (February 1984). "Sympathetic activity in benign familial tremor". Lancet. 1 (8374): 461–462. doi:10.1016/S0140-6736(84)91804-X. PMID 6142198. S2CID 36267406.
- ^ Wurtman RJ, Pohorecky LA, Baliga BS (June 1972). "Adrenocortical control of the biosynthesis of epinephrine and proteins in the adrenal medulla". Pharmacological Reviews. 24 (2): 411–426. PMID 4117970.
- ^ Wright A, Jones IC (June 1955). "Chromaffin tissue in the lizard adrenal gland". Nature. 175 (4466): 1001–1002. Bibcode:1955Natur.175.1001W. doi:10.1038/1751001b0. PMID 14394091. S2CID 36742705.
- ^ Coupland RE (April 1953). "On the morphology and adrenaline-nor-adrenaline content of chromaffin tissue". The Journal of Endocrinology. 9 (2): 194–203. doi:10.1677/joe.0.0090194. PMID 13052791.
- ^ a b c d Aronson JK (February 2000). ""Where name and image meet"—the argument for "adrenaline"". BMJ. 320 (7233): 506–509. doi:10.1136/bmj.320.7233.506. PMC 1127537. PMID 10678871.
- ^ European Pharmacopoeia 7.0 07/2008:2303
- ^ "Has adrenaline become a generic trademark?". genericides.org. Archived from the original on 1 May 2021.
- ^ "Naming human medicines – GOV.UK". www.mhra.gov.uk. 6 June 2019.
- ^ a b c d Rao Y (June 2019). "The First Hormone: Adrenaline". Trends in Endocrinology and Metabolism. 30 (6): 331–334. doi:10.1016/j.tem.2019.03.005. PMID 31064696. S2CID 144207341.
- ^ Shen H (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 4. ISBN 978-1-59541-101-3.
- ^ Arnall DA, Marker JC, Conlee RK, Winder WW (June 1986). "Effect of infusing epinephrine on liver and muscle glycogenolysis during exercise in rats". The American Journal of Physiology. 250 (6 Pt 1): E641–E649. doi:10.1152/ajpendo.1986.250.6.E641. PMID 3521311.
- ^ Raz I, Katz A, Spencer MK (March 1991). "Epinephrine inhibits insulin-mediated glycogenesis but enhances glycolysis in human skeletal muscle". The American Journal of Physiology. 260 (3 Pt 1): E430–E435. doi:10.1152/ajpendo.1991.260.3.E430. PMID 1900669.
- ^ a b Sircar S (2007). Medical Physiology. Thieme Publishing Group. p. 536. ISBN 978-3-13-144061-7.
- ^ Vasudevan NT, Mohan ML, Goswami SK, Naga Prasad SV (November 2011). "Regulation of β-adrenergic receptor function: an emphasis on receptor resensitization". Cell Cycle. 10 (21): 3684–3691. doi:10.4161/cc.10.21.18042. PMC 3266006. PMID 22041711.
- ^ Berg JM, Tymoczko JL, Stryer L (2002). "Epinephrine and Glucagon Signal the Need for Glycogen Breakdown". Biochemistry (5th ed.). New York: W.H. Freeman. ISBN 0-7167-3051-0.
- ^ "Guideline 11.5: Medications in Adult Cardiac Arrest" (PDF). Australian Resuscitation Council. December 2010. Retrieved 7 March 2015.
- ^ Chang YT, Huang WC, Cheng CC, Ke MW, Tsai JS, Hung YM, et al. (February 2020). "Effects of epinephrine on heart rate variability and cytokines in a rat sepsis model". Bosnian Journal of Basic Medical Sciences. 20 (1): 88–98. doi:10.17305/bjbms.2018.3565. PMC 7029199. PMID 29984678.
- ^ Burnett AM, Segal N, Salzman JG, McKnite MS, Frascone RJ (August 2012). "Potential negative effects of epinephrine on carotid blood flow and ETCO2 during active compression-decompression CPR utilizing an impedance threshold device". Resuscitation. 83 (8): 1021–1024. doi:10.1016/j.resuscitation.2012.03.018. PMID 22445865.
- ^ Raymondos K, Panning B, Leuwer M, Brechelt G, Korte T, Niehaus M, et al. (May 2000). "Absorption and hemodynamic effects of airway administration of adrenaline in patients with severe cardiac disease". Annals of Internal Medicine. 132 (10): 800–803. doi:10.7326/0003-4819-132-10-200005160-00007. PMID 10819703. S2CID 12713291.
- ^ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 545–547. ISBN 978-0-9626523-7-0.
- ^ von Bohlen und Haibach O, Dermietzel R (2006). Neurotransmitters and Neuromodulators: Handbook of Receptors and Biological Effects. Wiley-VCH. p. 125. ISBN 978-3-527-31307-5.
- ^ Kirshner N, Goodall M (June 1957). "The formation of adrenaline from noradrenaline". Biochimica et Biophysica Acta. 24 (3): 658–659. doi:10.1016/0006-3002(57)90271-8. PMID 13436503.
- ^ Axelrod J (May 1962). "Purification and properties of phenylethanolamine-N-methyl transferase". The Journal of Biological Chemistry. 237 (5): 1657–1660. doi:10.1016/S0021-9258(19)83758-4. PMID 13863458.
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ Nelson L, Cox M (2004). Lehninger Principles of Biochemistry (4th ed.). New York: Freeman. p. 908. ISBN 0-7167-4339-6.
- ^ "SLC18 family of vesicular amine transporters". Guide to Pharmacology. IUPHAR/BPS. Retrieved 21 August 2015.
- ^ Oanca G, Stare J, Mavri J (December 2017). "How fast monoamine oxidases decompose adrenaline? Kinetics of isoenzymes A and B evaluated by empirical valence bond simulation". Proteins. 85 (12): 2170–2178. doi:10.1002/prot.25374. PMID 28836294. S2CID 5491090.
- ^ Szablewski L (2011). Glucose Homeostasis and Insulin Resistance. Bentham Science Publishers. p. 68. ISBN 9781608051892.
- ^ Skalski JH, Kuch J (April 2006). "Polish thread in the history of circulatory physiology". Journal of Physiology and Pharmacology. 57 (Suppl 1): 5–41. PMID 16766800.
- ^ Bates WH (16 May 1896). "The Use of Extract of Suprarenal Capsule in the Eye". New York Medical Journal: 647–650. Retrieved 7 March 2015.
Read before the Section in Ophthalmology of the New York Academy of Medicine, 20 April 1896
- ^ Takamine J (1901). "The isolation of the active principle of the suprarenal gland". The Journal of Physiology. Great Britain: Cambridge University Press: xxix–xxx.
- ^ Bennett MR (June 1999). "One hundred years of adrenaline: the discovery of autoreceptors". Clinical Autonomic Research. 9 (3): 145–159. doi:10.1007/BF02281628. PMID 10454061. S2CID 20999106.
- ^ a b Ball CM, Featherstone PJ (May 2017). "The early history of adrenaline". Anaesthesia and Intensive Care. 45 (3): 279–281. doi:10.1177/0310057X1704500301. PMID 28486885.
- ^ Arthur G (May 2015). "Epinephrine: a short history". The Lancet. Respiratory Medicine. 3 (5): 350–351. doi:10.1016/S2213-2600(15)00087-9. PMID 25969360.
- ^ Zuckerman M (2007). Sensation seeking and risky behavior. Vol. 14. Washington, DC: American Psychological Association. pp. 41–52. doi:10.1016/0191-8869(93)90173-Z. ISBN 9781591477389.
{{cite book}}:|journal=ignored (help) - ^ a b Jänig W (6 July 2006). The integrative action of the autonomic nervous system: neurobiology of homeostasis. England: Cambridge University Press. pp. 143–146. ISBN 9780521845182.
- ^ Deane WH, Rubin BL (1964). "Absence of adrenal meduallary secretions". The Adrenocortical Hormones Their Origin – Chemistry Physiology and Pharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg. p. 105. ISBN 9783662131329.
- ^ Frankenhaeuser M, Jarpe G, Matell G (February 1961). "Effects of intravenous infusions of adrenaline and noradrenaline on certain psychological and physiological functions". Acta Physiologica Scandinavica. 51 (2–3): 175–186. doi:10.1111/j.1748-1716.1961.tb02126.x. PMID 13701421.
- ^ Wise J (28 December 2009). "When Fear Makes Us Superhuman". Scientific American. Retrieved 25 August 2015.
- ^ Wise J (8 December 2009). Extreme Fear: The Science of Your Mind in Danger (1st ed.). New York: Palgrave Macmillan. ISBN 9780230101807.
External links
[edit] Media related to Epinephrine at Wikimedia Commons
Media related to Epinephrine at Wikimedia Commons- "U.S. National Library of Medicine: Drug Information Portal – Epinephrine". Archived from the original on 14 December 2019.
- Adrenaline
- Anxiety
- Alpha-adrenergic agonists
- Beta-adrenergic agonists
- Bronchodilators
- Carbonic anhydrase activators
- Cardiac stimulants
- Catecholamines
- Hormones of the hypothalamus-pituitary-adrenal axis
- Hormones of the suprarenal medulla
- Neurotransmitters
- Norepinephrine releasing agents
- Stress (biology)
- Sympathomimetic amines
- Chemical substances for emergency medicine
- Phenylethanolamines
- Human metabolites
- Stress hormones



