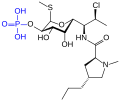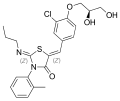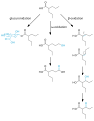User:Anypodetos/Chemical formulae
Appearance
These are the chemical structure drawings I created with BKchem, BIOVIA Draw and Inkscape. All images are scalable vector graphics and (unless noted otherwise) released into the public domain:
New chemical formulae
[edit]-
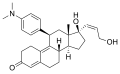
A 276575, a SEGRA
-
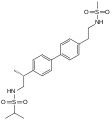
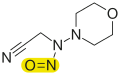
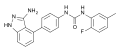
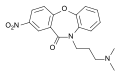
Acetylamifampridine
-
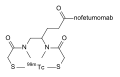
ACT-132577, the active metabolite of macitentan
-
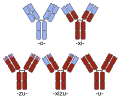
Antibody with CDRs
-
Afatinib binding to EGFR
-
Alfatradiol compared to estradiol
-
Anemoninsäure
-
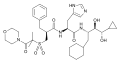
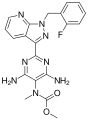
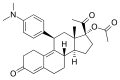
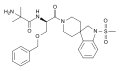
Ambrisentan (stereochemistry)
-
BIBF 1202, the main metabolite of nintedanib
-
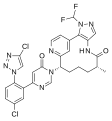
Branaplam keto-enol tautomerism
-
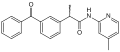
Bromfenac cyclic amide
-
Carbamazepine metabolism
-
Catumaxomab mechanism
-
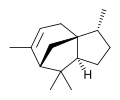
(−)-α-Cedrene
-
(+)-β-Cedrene
-
Version with CDRs
-
Activation of clopidogrel
-
Colestilan, alternative structure
-
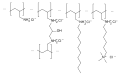
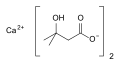
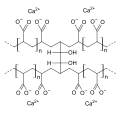
Colestipol, alternative structure
-
Dabigatran etexilate
-
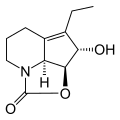
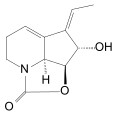
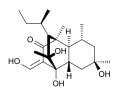
Dihydrostreptazolin
-
Enalkiren (another orientation)
-
Engineered monoclonal antibodies
-
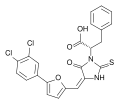
Epimerox, compound 1
-
Epimerox, compound 2
-
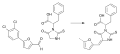
Epimerox synthesis
-
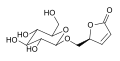
Eritoran acid
-
Eritoran tetrasodium
-
FR for C-glycosides
-
Nitrogen analog of the FR
-
Other tautomer of foliglurax
-
Heavy-chain and common antibody
-
Hydroxyethyl succinimide, a metabolite of diroximel fumarate
-
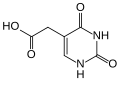
Hydroxylornoxicam, the metabolite of lornoxicam
-
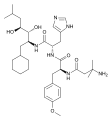
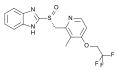
ID-11614 and ID-20219, metabolites of lurasidone
-
ID-14283, active metabolite of lurasidone
-
(R)-D-Isoglutamine
-
(S)-L-Isoglutamine
-
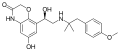
LBQ657, the active metabolite of sacubitril
-
Lenvatinib metabolism
-
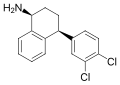
Levocetirizine (stereochemistry)
-
Levodopa activation and inactivation
-
Levofolinic acid (stereochemistry)
-
Lorlatinib metabolism
-
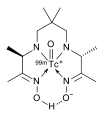
Chelator of lutetium (177Lu) lilotomab satetraxetan
-
Comparison of cis and trans mefway
-
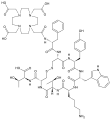
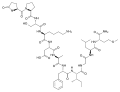
Mertansine plus mab
-
Mertansine plus mab, colour coded
-
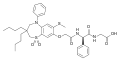
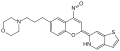
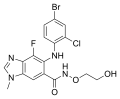
MK-5932, a SEGRA
-
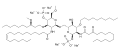
Monomethyl auristatin E-mab-conjugate
-
Monomethyl auristatin F-mab-conjugate
-
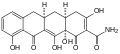
Morphine (stereochemistry)
-
MRE 269, the active metabolite of selexipag
-
Netupitant metabolites
-
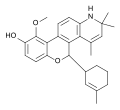
Octahydrophenanthrene-2,7-diol type SEGRA
-
Synthesis of olaflur
-
Opicapone and metabolites
-
Oteracil potassium
-
Palonosetron metabolites
-
Pegaptanib sodium
-
Phosphorothioate DNA of an antisense oligonucleotide
-
Phosphorothioate RNA of an antisense oligonucleotide
-
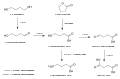
Activation of prasugrel
-
Active metabolite of prasugrel
-
Prostamide E2
-
Quadroma antibodies
-
RU 24858, a SEGRA
-
SIN-1A, the active metabolite of molsidomine
-
scFv, polyvalent
-
Sodium alkyl sulfates
-
Spilanthol from Acmella oleracea
-
Another Acmella oleracea amide
-
Tafluprost metabolism
-
E-Teriflunomide
-
Ticagrelor metabolite
-
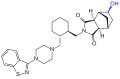
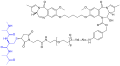
TL 12-186, a proteolysis targeting chimera
-
Tofogliflozin
-
Tofogliflozin monohydrate
-
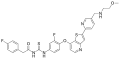
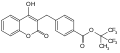
Tolcapone metabolism
-
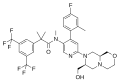
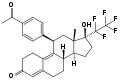
Trifluoroisopropanolamine / amide type SEGRAs
-
Valproic acid metabolism
-
Vandetanib metabolism
-
ZK 216348, a SEGRA
Vectorised chemical formulae
[edit]-
Azathioprine metabolism (added formulae)
-
Betaenones A, B and C
-
Betaenone B biosynthesis
-
Butenyl-methyl-L-threonine biosynthesis
-
The PDE3 inhibitor CI-930 (stereochemistry)
-
CI-930 with its heterocycle – phenyl – imidazole pattern
-
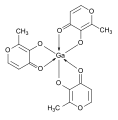
Cisplatin synthesis
-
Cisplatin and transplatin
-
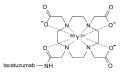
DOTA (tetraxetan)
-
Eledoisin (stereochemistry)
-
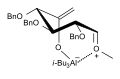
Ferrier carbocyclization (FCC)
-
Mechanism of the FCC
-
FCC continued
-
Sinaÿ's modification of the FCC
-
Proposed transition state for Sinaÿ's modification of the FCC
-
Planar chiral ferrocene derivative
-
Gitoformate (stereochemistry)
-
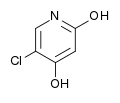
Metabolic pathways of gamma-hydroxybutyric acid
-
HU-320 (stereochemistry)
-
Incarvillateine (stereochemistry)
-
Levobetaxolol (stereochemistry)
-
Lornoxicam (tautomerism)
-
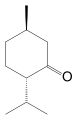
(-)-Menthone
-
(+)-Menthone
-
Meribendan (stereochemistry)
-
Metapramine (stereochemistry)
-
Methylenedioxy group
-
2-Methyl-MDA (stereochemistry)
-
Octamoxin (stereochemistry)
-
Synthesis of rapamycin from prerapamycin
-
"Protonated" rhodocene
-
Proton pump inhibitors mechanism
-
Proton pump inhibitors structure
-
PTMEG synthesis
-
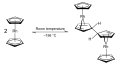
Rhodocene dimerisation
-
Decaisopropylrhodicinium hexafluorophosphate synthesis
-
Staggered ferrocene and eclipsed ruthenocene
-
Synthesis of the 1-cobaltocenyl-1'-rhodocenylferrocene cation
-
Bi- and ter-metallocenes containing rhodocenyl groups
-
Synthesis of the 1,2,3-tri-tert-butylrhodocenium cation
-
Rivaroxaban (stereochemistry)
-
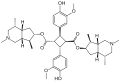
Sofosbuvir mechanism
-
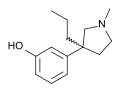
SRT2183 (stereochemistry)
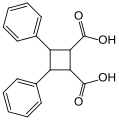
-
Truxillic and truxinic acid stereochemistry