Salsalate
 | |
| Clinical data | |
|---|---|
| Trade names | Disalcid, Salflex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682880 |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.208 |
| Chemical and physical data | |
| Formula | C14H10O5 |
| Molar mass | 258.229 g·mol−1 |
| | |
Salsalate is a medication that belongs to the salicylate and nonsteroidal anti-inflammatory drug (NSAID) classes.
Salsalate is the generic name of a prescription drug marketed under the brandnames Mono-Gesic, Salflex, Disalcid, and Salsitab. Other generic and brand name formulations may be available.[2]
Mechanism of action
[edit]Relative to other NSAIDs, salsalate has a weak inhibitory effect on the cyclooxygenase enzyme and decreases the production of several proinflammatory chemical signals such as interleukin-6, TNF-alpha, and C-reactive protein.[3]
The mechanism through which salsalate is thought to reduce the production of these inflammatory chemical signals is through the inhibition of IκB kinase resulting in decreased action of NF-κB genes.[3][4][5] This mechanism is thought to be responsible for salsalate's insulin-sensitizing and blood sugar lowering properties.[4]
Medical uses
[edit]Salsalate may be used for inflammatory disorders such as rheumatoid arthritis or noninflammatory disorders such as osteoarthritis.[3][6]
Safety
[edit]The risk of bleeding is a common concern with use of the NSAID class of medications. However, the bleeding risk associated with salsalate is lower than that associated with aspirin use.[4]
Research
[edit]Salsalate has been proposed for the prevention and treatment of type 2 diabetes mellitus due to its ability to lower insulin resistance associated with inflammation and may be useful in prediabetes.[3] However, the use of salsalate to prevent the progression from prediabetes to type 2 diabetes mellitus has received limited study.[3]
History
[edit]This section is missing information about first known synthesis and commercialization — who decided to stick two salicylic acid molecules together?. (January 2023) |
Salsalate had been suggested as possible treatment for diabetes as early as 1876.[3][7][8]
Synthesis
[edit]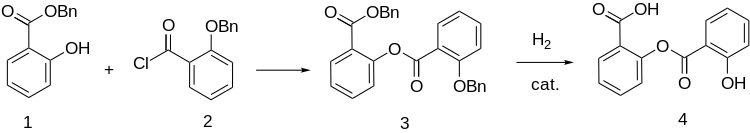
References
[edit]- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Salsalate". drugs.com.
- ^ a b c d e f Anderson K, Wherle L, Park M, Nelson K, Nguyen L (June 2014). "Salsalate, an old, inexpensive drug with potential new indications: a review of the evidence from 3 recent studies". American Health & Drug Benefits. 7 (4): 231–5. PMC 4105730. PMID 25126374.
- ^ a b c Esser N, Paquot N, Scheen AJ (March 2015). "Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease". Expert Opinion on Investigational Drugs (Review). 24 (3): 283–307. doi:10.1517/13543784.2015.974804. PMID 25345753. S2CID 23674166.
- ^ Ridker PM, Lüscher TF (July 2014). "Anti-inflammatory therapies for cardiovascular disease". European Heart Journal. 35 (27): 1782–91. doi:10.1093/eurheartj/ehu203. PMC 4155455. PMID 24864079.
- ^ Hardie DG (July 2013). "AMPK: a target for drugs and natural products with effects on both diabetes and cancer". Diabetes. 62 (7): 2164–72. doi:10.2337/db13-0368. PMC 3712072. PMID 23801715.
- ^ Powell K (May 2007). "Obesity: the two faces of fat". Nature. 447 (7144): 525–7. Bibcode:2007Natur.447..525P. doi:10.1038/447525a. PMID 17538594. S2CID 28974642.
- ^ Ebstein W (1876). "Zur therapie des diabetes mellitus, insbesondere uber die anwendung des salicylsauren natron bei demselben". Berliner Klinische Wochenschrift. 13: 337–340.
- ^ Cavallito CJ, Buck JS (1943). "Synthesis of Phenolic Acid Esters. I. Depsides1". Journal of the American Chemical Society. 65 (11): 2140–2142. doi:10.1021/ja01251a034.
- ^ Baker W, Ollis WD, Zealley TS (1951). "42. Eight- and higher-membered ring compounds. Part II. Di-, tri-, tetra-, and hexa-salicylides". Journal of the Chemical Society (Resumed): 201. doi:10.1039/JR9510000201.
- ^ DE 211403, "Verfarhen zur Darstellung einer kristallisierten Salicylosalicylsäure aus Salicylsäure oder ihrne Salzen [Process for preparing a crystallized salicylosalicylic acid from salicylic acid or its salts]", published 1909-06-25, assigned to C.F. Boehringer & Söhne
- ^ DE 214044, "Verfarhen zur Darstellung einer kristallisierten Salicylosalicylsäure [Process for preparing a crystallized salicylosalicylic acid]", published 1909-09-20, assigned to C.F. Boehringer & Söhne
