Picenadol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H25NO |
| Molar mass | 247.382 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Picenadol (LY-97435) is a 4-phenylpiperidine derivative that is an opioid analgesic drug developed by Eli Lilly in the 1970s.[1]
Picenadol is an effective analgesic with similar efficacy to pethidine (meperidine). It has been investigated for some applications such as obstetrics[2] and dentistry,[3] but never commercialised.
It is unusual in that one enantiomer is a pure μ-opioid agonist, while the other is an antagonist.[4] The (3S,4R) isomer is the agonist, while (3R,4S) is antagonist.[5] This means that the racemic mix of the two enantiomers is a mixed agonist-antagonist, with relatively low abuse potential, and little of the κ-opioid activity that tends to cause problems with other opioid mixed agonist-antagonists such as pentazocine.[6]
Synthesis
[edit]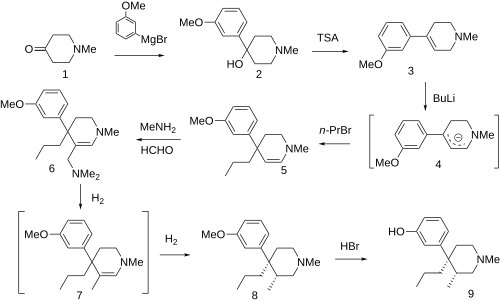

See also
[edit]References
[edit]- ^ US 4081450, Zimmerman DM, "1,3,4-Trisubstituted-4-arylpiperidines and their preparation", issued 28 April 1978, assigned to Eli Lilly & Company
- ^ Sherline DM (October 1983). "Picenadol (LY 150720) compared with meperidine and placebo for relief of post-cesarean section pain: a randomized double-blind study". American Journal of Obstetrics and Gynecology. 147 (4): 404–6. doi:10.1016/s0002-9378(16)32234-7. PMID 6624809.
- ^ Goldstein DJ, Brunelle RL, George RE, Cooper SA, Desjardins PJ, Gaston GW, Jeffers GE, Gallegos LT, Reynolds DC (1994). "Picenadol in a large multicenter dental pain study". Pharmacotherapy. 14 (1): 54–9. doi:10.1002/j.1875-9114.1994.tb02789.x. PMID 8159602. S2CID 24644644.
- ^ Leander JD, Zimmerman DM (December 1983). "Effects of picenadol and its agonist and antagonist isomers on schedule-controlled behavior". The Journal of Pharmacology and Experimental Therapeutics. 227 (3): 671–5. PMID 6655562.
- ^ Froimowitz M, Cody V. Absolute configurations and conformations of the opioid agonist and antagonist enantiomers of picenadol. Chirality. 1995;7(7):518-25.
- ^ Zimmerman DM, Smits SE, Hynes MD, Cantrell BE, Leander JD, Mendelsohn LG, Nickander R (February 1985). "Picenadol". Drug and Alcohol Dependence. 14 (3–4): 381–401. doi:10.1016/0376-8716(85)90069-9. PMID 2986931.
- ^ US 4499274, Feth G, Mills JE, "Process for preparation of substituted formamidine and substituted N-iminomethyl piperidine", published 1985-02-12, assigned to McNeil Lab Inc.
- ^ Martinelli MJ, Peterson BC (1990). "A concise, stereoselective synthesis of picenadol". Tetrahedron Letters. 31 (38): 5401–5404. doi:10.1016/S0040-4039(00)97857-2.
