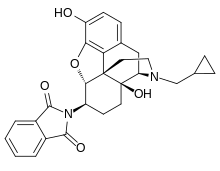
Naltalimide
Appearance
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H28N2O5 |
| Molar mass | 472.541 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Naltalimide (INN) (code name TRK-130, formerly TAK 363) is a novel, centrally-acting opioid drug which is under development by Takeda and Toray for the treatment of overactive bladder/urinary incontinence.[1][2] It acts as a potent and selective partial agonist of the μ-opioid receptor (Ki = 0.268 nM, EC50 = 2.39 nM, Emax = 66.1%) over the δ-opioid (Ki = 121 nM, EC50 = 26.1 nM, Emax = 71.0%) and κ-opioid receptors (Ki = 8.97 nM, EC50 = 9.51 nM, Emax = 62.6%).[1] Notably, naltalimide somehow appears to lack certain undesirable side effects such as constipation seen with other μ-opioid receptor agonists such as morphine.[1] It enhances bladder storage via suppression of the afferent limb of the micturition reflex pathway.[1]
References
[edit]- ^ a b c d Fujimura M, Izumimoto N, Momen S, Yoshikawa S, Kobayashi R, Kanie S, et al. (September 2014). "Characteristics of TRK-130 (Naltalimide), a novel opioid ligand, as a new therapeutic agent for overactive bladder". The Journal of Pharmacology and Experimental Therapeutics. 350 (3): 543–51. doi:10.1124/jpet.114.214031. PMID 24928951. S2CID 2238176.
- ^ "TRK 130 Takeda Toray licensing agreement". HighBeam. Archived from the original on 2016-03-09.
