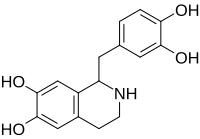
Tetrahydropapaveroline
Appearance
| |
| Names | |
|---|---|
| IUPAC name
1-[(3,4-dihydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol
| |
| Other names
Norlaudanosoline; Tetrahydroxypapaveroline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.158.898 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H17NO4 | |
| Molar mass | 287.315 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrahydropapaveroline (norlaudanosoline) is a benzyltetrahydroisoquinoline alkaloid.[1]
It can be formed in trace amounts in the brain by a condensation reaction of dopamine and dopaldehyde (a metabolite of dopamine).[1][2]
It inhibits dopamine uptake within the cerebral cortex.[3]
References
[edit]- ^ a b Richter, Derek (14 October 2016). Addiction and Brain Damage. Routledge. p. 24. ISBN 978-1-315-45403-0.
- ^ RD Myers; CL Melchior (29 April 1977). "Alcohol drinking: abnormal intake caused by tetrahydropapaveroline in brain". Science. 196 (4289): 554–556. Bibcode:1977Sci...196..554M. doi:10.1126/science.557839. PMID 557839.
- ^ Okada, T.; Shimada, S.; Sato, K.; Kotake, Y.; Kawai, H.; Ohta, S.; Tohyama, M.; Nishimura, T. (January 1998). "Tetrahydropapaveroline and its derivatives inhibit dopamine uptake through dopamine transporter expressed in HEK293 cells". Neuroscience Research. 30 (1): 87–90. doi:10.1016/s0168-0102(97)00121-1. ISSN 0168-0102. PMID 9572583.
