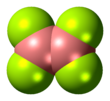
Diboron tetrafluoride
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Diboron tetrafluoride | |||
| Systematic IUPAC name
Tetrafluorodiborane(4) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| B2F4 | |||
| Molar mass | 97.61 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 4.3 kg/m3 (gas) | ||
| Melting point | −56 °C (−69 °F; 217 K) | ||
| Boiling point | −34 °C (−29 °F; 239 K) | ||
| Thermochemistry | |||
Heat capacity (C)
|
79.1 J/mol K | ||
Std molar
entropy (S⦵298) |
317.3 J/mol K | ||
Std enthalpy of
formation (ΔfH⦵298) |
-1440.1 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
-1410.4 kJ/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Diboron tetrafluoride is the inorganic compound with the formula (BF2)2. A colorless gas, the compound has a halflife of days at room temperature. It is the most stable of the diboron tetrahalides,[1] and does not appreciably decompose under standard conditions.[2]
Structure and bonding
[edit]Diboron tetrafluoride is a planar molecule with a B-B bond distance of 172 pm.[1] Although it is electron-deficient, the unsaturated boron centers are stabilized by pi-bonding with the terminal fluoride ligands. The compound is isoelectronic with oxalate.
Synthesis and reactions
[edit]Diboron tetrafluoride can be formed by treating boron monofluoride with boron trifluoride at low temperatures, taking care not to form higher polymers.[3] Alternatively, diboron tetrachloride can be fluorinated with antimony trifluoride.[2]
Addition of diboron tetrafluoride to Vaska's complex was employed to produce an early example of a transition metal boryl complex:[4]
- 2 B2F4 + IrCl(CO)(PPh3)2 → Ir(BF2)3(CO)(PPh3)2 + ClBF2
Historical literature
[edit]- Louis Trefonas and William N. Lipscomb (1958). "Crystal and Molecular Structure of Diboron Tetrafluoride, B2F4". J. Chem. Phys. 28 (1): 54–55. Bibcode:1958JChPh..28...54T. doi:10.1063/1.1744079.
- Gayles, J. N.; Self, J. (1964). "Infrared Spectrum of Diboron Tetrafluoride in the Gaseous and Solid States". Journal of Chemical Physics. 40 (12): 3530–3539. Bibcode:1964JChPh..40.3530G. doi:10.1063/1.1725048.
- A. K. Holliday; F. B. Taylor (1964). "Diboron tetrafluoride. Part II. Reactions with some oxides and organometallic compounds". J. Chem. Soc.: 2731–2734. doi:10.1039/JR9640002731.
- Vernon H. Dibeler; Susan K. Liston (1968). "Mass-spectrometric study of photoionization. XII. Boron trifluoride and diboron tetrafluoride". J. Chem. Soc. 7 (9): 1742–1746. doi:10.1021/ic50067a010.
References
[edit]- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Arthur Finch and Hermann Irving Schlesinger (1958). "Diboron Tetrafluoride". J. Am. Chem. Soc. 80 (14): 3573–3574. doi:10.1021/ja01547a020.
- ^ Timms, P. L. (1972). "Low Temperature Condensation of High Temperature Species as a Synthetic Method". Advances in Inorganic Chemistry and Radiochemistry. Academic Press: 143. doi:10.1016/S0065-2792(08)60006-0. ISBN 0-12-023614-1.
- ^ Neeve, Emily C.; Geier, Stephen J.; Mkhalid, Ibraheem A. I.; Westcott, Stephen A.; Marder, Todd B. (2016). "Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse". Chemical Reviews. 116 (16): 9091–9161. doi:10.1021/acs.chemrev.6b00193. hdl:1807/78811. PMID 27434758.