Mephedrone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 4-methyl-N-methylcathinone; 2-methylamino-1-p-tolylpropan-1-one[1] |
| Routes of administration | Oral, insufflation, IV, rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.189.720 |
| Chemical and physical data | |
| Formula | C11H15NO |
| Molar mass | 177.247 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mephedrone, also known as 4-methylmethcathinone, 4-MMC, and 4-methylephedrone, is a synthetic stimulant drug belonging to the amphetamine and cathinone classes. It is commonly referred to by slang names such as drone,[5] M-CAT,[6] White Magic,[7] meow meow, and bubble.[8] Chemically, it is similar to the cathinone compounds found in the Khat plant, native to eastern Africa.[5][9]
Mephedrone is typically found in tablet or crystal form,[10] and users may swallow, snort, or inject it. Its effects are similar to those of MDMA, amphetamines, and cocaine, producing euphoria and increased sociability. Mephedrone is rapidly absorbed, with a half-life of about 2 hours, and is primarily metabolized by CYP2D6 enzymes. Its effects are dose-dependent. Side effects can include cardiovascular changes[4]: 13 and anxiety.[11][12]
Mephedrone was first synthesised in 1929 but remained relatively obscure until it was rediscovered around 1999–2000. At that time, it was legal to produce and possess in many countries. By 2000, mephedrone was available for sale on the internet. By 2008, law enforcement agencies had become aware of the substance, and by 2010, it had been reported in most European countries, with significant prevalence in the United Kingdom. Mephedrone was first made illegal in Israel in 2008, followed by Sweden later that year. By 2010, many European countries had banned the substance, and in December of that year, the European Union ruled it illegal. In Australia, New Zealand, and the United States, it is considered an analog of other illegal drugs and can be controlled under laws similar to the US Federal Analog Act. In September 2011, the US temporarily classified mephedrone as a Schedule I drug, with the classification taking effect in October 2011. This was made permanent in July 2012 with the passage of the Synthetic Drug Abuse Prevention Act (SDAPA).[13]
Uses
[edit]Recreational
[edit]Users have reported that mephedrone causes euphoria, stimulation, an enhanced appreciation for music, an elevated mood, decreased hostility, improved mental function and mild sexual stimulation; these effects are similar to the effects of cocaine, amphetamines and MDMA, and last different lengths of time depending on the way the drug is taken. Of 70 Dutch users of mephedrone, 58 described it as an overall pleasant experience and 12 described it as an unpleasant experience.[14] In a survey of UK users who had previously taken cocaine, most users found it produced a better-quality and longer-lasting high and was less addictive. The users were also asked to compare the "risk", and they answered that it was equal.[15] A study of users in Northern Ireland found they did not equate the fact that mephedrone was legal with it being safe to use. This was contrary to another study in New Zealand, where users of benzylpiperazine thought that because it was legal, it was safe.[16]
Available forms
[edit]Mephedrone can come in the form of capsules, tablets or white powder that users may swallow, snort, inject, smoke or use rectally.[4]: 12 [15][17] When taken orally, users reported they could feel the effects within 15–45 minutes; when snorted, the effects were felt within minutes and peaked within half an hour. The effects last for between two and three hours when taken orally or nasally, but only half an hour if taken intravenously.[4]: 12 It is sometimes sold mixed with methylone in a product called bubbles in the UK[18] and also mixed with other cathinones, including ethcathinone, butylone, fluoromethcathinone and methedrone.[4]: 9
Purity
[edit]One published study that analysed samples of mephedrone bought using the internet in the UK in 2010 found it was racemic (a mixture of both stereoisomers) and of high purity.[19] An unpublished study of six samples also ordered off the internet in the UK in 2010 found they contained very few organic impurities.[20] Four products sold in Irish head shops were tested in 2010 and were found to contain between 82% and 14% mephedrone, with some products containing benzocaine and caffeine.[21]
Adverse effects
[edit]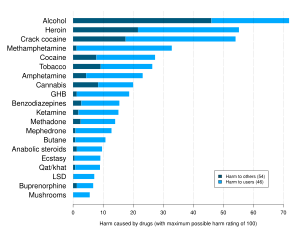
Short-term effects
[edit]The EMCDDA reported mephedrone can cause various unintended side effects including: dilated pupils,[23] poor concentration, teeth grinding, problems focusing visually, poor short-term memory, hallucinations, delusions, and erratic behaviour.[4]: 13 [12] They noted the most severe effects appear anecdotally to be linked with high doses or prolonged use, and the effects may be due to users taking other intoxicants at the same time. Other effects users in internet forums have noted include changes in body temperature, increased heart rate, breathing difficulties, loss of appetite, increased sweating, discolouration of extremities, anxiety, paranoia and depression.[4]: 13 When snorted, it can also cause nose bleeds and nose burns.[4]: 13 [24] A survey conducted by the UK National Addiction Centre found 67% of mephedrone users experienced sweating, 51% suffered from headaches, 43% from heart palpitations, 27% from nausea and 15% from cold or blue fingers,[25] indicative of vasoconstriction occurring.[26] Doctors at Guy's Hospital in London reported that of 15 patients they treated after taking mephedrone in 2009, 53% were agitated, 40% had increased heart rates, 20% had systolic hypertension and 20% had seizures; three required treatment with benzodiazepines, predominantly to control their agitation. They reported none of their patients suffered from cold or blue peripheries, contrary to other reports. Nine of the 15 of patients had a Glasgow coma scale (GCS) of 15, indicating they were in a normal mental state, four had a GCS below 8, but these patients all reported using a central nervous system depressant, most commonly GHB, with mephedrone. The patients also reported polydrug use of a variety of compounds.[27]
Neurotoxicity
[edit]Mephedrone has been found to be a monoaminergic neurotoxin in animals and induces serotonergic neurotoxicity.[28][29][30][31] Although some studies in animal models reported no damage to dopamine nerve endings in the striatum and no significant changes in brain monoamine levels, some others suggested a rapid reduction in serotonin transporter (SERT) and dopamine transporter (DAT) function.[28] Persistent serotonergic deficits were observed after binge like treatment in a warm environment and in both serotonergic and dopaminergic nerve endings at high ambient temperature. Oxidative stress cytotoxicity and an increase in frontal cortex lipid peroxidation were also reported.[30][32] Although mephedrone has been found to be a monoaminergic neurotoxin, in one study, moderate doses of MDMA produced serotonergic neurotoxicity in rodents whereas mephedrone and methylone did not do so, suggesting that cathinones like mephedrone may be less neurotoxic than their corresponding amphetamine counterparts like MDMA.[33][34]
Reinforcement disorders
[edit]There have been reports of users craving mephedrone, suggesting it may be addictive.[4]: 13 [14]
Overdose
[edit]Toxicity
[edit]In 2009, one case of sympathomimetic toxicity was reported in the UK after a person took 0.2 g of mephedrone orally, and after this did not achieve the desired effect, intramuscularly injected 3.8 g mixed with water into his thighs. Shortly afterwards, the user "developed palpitations, blurred tunnel vision, chest pressure and sweating". The patient was treated with 1 mg of lorazepam and the sympathomimetic features decreased and the user was discharged within six hours of arrival.[35] One case of serotonin syndrome has been reported, where the patient was already prescribed fluoxetine and olanzapine, and then took 40 tablets containing mephedrone in one night. He was treated with lorazepam and discharged 15 hours after admission.[36] Both enantiomers of methcathinone, which differs only in the lack of the methyl group on the aryl ring when compared to mephedrone, have been shown to be toxic to rat dopamine neurons, and the S-enantiomer was also toxic against serotonin neurons. Simon Gibbons and Mire Zloh of the School of Pharmacy, University of London stated, based on the chemical similarities between methcathinone and mephedrone, "it is highly likely that mephedrone will display neurotoxicity".[19] However, Brunt and colleagues stated, "extreme caution" should be used when inferring the toxicity of mephedrone from methcathinone, noting some of the toxicity associated with methcathinone is due to manganese impurities related to its synthesis, rather than the compound itself. They concluded more experimental research is needed to investigate the toxicity of mephedrone.[14]
Doctors who treated a 15-year-old female suffering from mephedrone intoxication suggested in The Lancet that, like MDMA, mephedrone may promote serotonin-mediated release of antidiuretic hormone, resulting in hyponatraemia and an altered mental state.[37] In another case, a 19-year-old male was admitted to hospital suffering from inflammation of the heart, 20 hours after taking one gram of mephedrone. The doctors treating the patient stated it was caused by either a direct toxic effect of mephedrone on the heart muscle, or by an immune response.[38] One case of acquired methaemoglobinaemia, where a patient had "bluish lips and fingers", has also been reported, after the user snorted one gram of mephedrone. The patient started to recover after arriving at the hospital and it was not necessary to administer any medication.[39]
Deaths
[edit]Sweden
[edit]In 2008, an 18-year-old Swedish woman died in Stockholm after taking mephedrone. The newspaper Svenska Dagbladet reported the woman went into convulsions and turned blue in the face.[40] Doctors reported she was comatose and suffering from hyponatremia and severe hypokalemia; the woman died one and a half days after the onset of symptoms. An autopsy showed severe brain swelling.[41] Mephedrone was scheduled to be classified as a "dangerous substance" in Sweden even before the woman's death at Karolinska University Hospital on 14 December, but the death brought more media attention to the drug. The possession of mephedrone became classified as a criminal offence in Sweden on 15 December 2008.[40]
United Kingdom
[edit]In 2010, unconfirmed reports speculated about the role mephedrone has played in the deaths of several young people in the UK. By July 2010, mephedrone had been alleged to be involved in 52 fatalities in the UK, but detected in only 38 of these cases. Of the nine that coroners had finished investigating, two were caused directly by mephedrone.[42] The first death reported to be caused by mephedrone use was that of 46-year-old, John Sterling Smith,[43] who had underlying health problems and repeatedly injected the drug.[44] A report in Forensic Science International in August 2010 stated mephedrone intoxication has been recorded as the cause of death in two cases in Scotland. Post mortem samples showed the concentration of mephedrone in their blood was 22 mg/L in one case and 3.3 mg/L in the other.[45] The death of a teenager in the UK in November 2009 was widely reported as being caused by mephedrone, but a report by the coroner concluded she had died from natural causes.[46] In March 2010, the deaths of two teenagers in Scunthorpe were widely reported by the media to be caused by mephedrone. Toxicology reports showed the teenagers had not taken any mephedrone and had died as a result of consuming alcohol and the synthetic opioid agonist methadone.[44][47] According to Fiona Measham, a criminologist who is a member of the ACMD, the reporting of the unconfirmed deaths by newspapers followed "the usual cycle of 'exaggeration, distortion, inaccuracy and sensationalism'" associated with the reporting of recreational drug use.[48]
United States
[edit]Mephedrone has been implicated in the death of a 22-year-old man, who had also injected black tar heroin. Mephedrone was found in his blood at a concentration of 0.50 mg/L and in his urine at a concentration of 198 mg/L. The blood concentration of morphine, a metabolite of heroin, was 0.06 mg/L.[49] For comparison, the average blood morphine concentration resulting from deadly overdoses involving only heroin is around 0.34 mg/L.[50]
Pharmacology
[edit]Pharmacodynamics
[edit]Mephedrone acts as a potent substrate for monoamine transporters, particularly the dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET). It inhibits the reuptake of these neurotransmitters and promotes their release.[11] The drug induces a rapid and significant increase in extracellular levels of dopamine, serotonin, and norepinephrine. This effect is more pronounced for serotonin compared to dopamine, which distinguishes mephedrone from some other psychostimulants. The pharmacodynamic profile of mephedrone is often compared to MDMA and methamphetamine. Like MDMA, it has a greater effect on serotonin than dopamine release. Similar to methamphetamine, it causes rapid dopamine release.[11] Mephedrone shows significant affinity for various receptors, including 5-HT2A receptors, α2-adrenergic receptors, and trace amine-associated receptor 1 (TAAR1).[11]
Mephedrone administration leads to increased extracellular dopamine in the nucleus accumbens, elevated serotonin levels in the frontal cortex, and alterations in brain temperature.[11] These pharmacodynamic actions translate into various behavioral effects, including increased locomotor activity, rewarding effects (as measured by conditioned place preference), and potential for drug discrimination (similar to cocaine and MDMA).[11]
Pharmacokinetics
[edit]Mephedrone is rapidly absorbed and eliminated in humans. After oral or intranasal administration, peak plasma concentrations are typically reached within 0.5 to 1 hour.[11] The drug crosses the blood-brain barrier easily, with a brain-to-plasma ratio of approximately 1.85 in rats.[11] It has a relatively short half-life of approximately 2 hours in plasma and whole blood.[51] The drug and its metabolites can be detected in whole blood and plasma for up to 6 hours post-administration, with some metabolites persisting longer.[11][51]
Mephedrone exhibits enantioselective pharmacokinetics. The R-(+) enantiomer shows higher peak concentrations and a longer half-life compared to the S-(-) enantiomer.[11][52] The absolute bioavailability of mephedrone is relatively low, at about 10% in rats, suggesting a significant first-pass effect.[11] The percentage of mephedrone bound to plasma proteins is approximately 22%.[11]
These pharmacokinetic properties contribute to mephedrone's rapid onset of action, short duration of effects, and the tendency for users to engage in repeated dosing to maintain the desired effects.
Metabolism
[edit]Mephedrone undergoes extensive metabolism, primarily through the cytochrome P450 2D6 (CYP2D6) enzyme.[53][51]
The main phase I metabolic pathways include N-demethylation, reduction of the ketone moiety, and oxidation of the tolyl group.[51] Key metabolites identified in human plasma and urine include nor-mephedrone, dihydro-mephedrone, hydroxytolyl-mephedrone, and 4-carboxy-mephedrone, with 4-carboxy-mephedrone being the most abundant.[11][54]
Based on the analysis of rat and human urine by gas chromatography and mass spectrometry, mephedrone is thought to be metabolised by three phase 1 pathways. It can be demethylated to the primary amine (producing compounds 2, 3 and 5), the ketone group can be reduced (producing 3), or the tolyl group can be oxidised (producing 6). Both 5 and 6 are thought to be further metabolised by conjugation to the glucuronide and sulfate derivatives. Knowledge of the primary routes of metabolism should allow the intake of mephedrone to be confirmed by drug tests, as well as more accurate determination of the causes of side effects and potential for toxicity.[55]

Detection in body fluids
[edit]Mephedrone may be quantitated in blood, plasma or urine by gas chromatography-mass spectrometry or liquid chromatography-mass spectrometry to confirm a diagnosis of poisoning in hospitalised patients or to provide evidence in a medicolegal death investigation. Blood or plasma mephedrone concentrations are expected to be in a range of 50–100 μg/L in persons using the drug recreationally, >100 μg/L in intoxicated patients and >500 μg/L in victims of acute overdosage.[56][57]<
Chemistry
[edit]Appearance and odour
[edit]Mephedrone is a white substance. It is sold most commonly as crystals or a powder, but also in the form of capsules or pills.[58][59] It can have a distinctive odour, reported to range the smell of vanilla and bleach, stale urine, or electric circuit boards.[60] Synthesis byproducts or other contaminants are likely responsible for this, as the molecule itself is not an odorant.
Synthesis
[edit]Mephedrone can be synthesised in several ways. The simplest method, due to the availability of the compounds,[4]: 17 is to add 4-methylpropiophenone dissolved in glacial acetic acid to bromine, creating an oil fraction of 4'-methyl-2-bromopropiophenone. The oil fraction can then be dissolved in dichloromethane (CH2Cl2) and drops of the solution added to another solution of CH2Cl2-containing methylamine hydrochloride and triethylamine. Hydrochloric acid (HCl) is then added and the aqueous layer is removed and turned alkaline using sodium hydroxide before the amine is extracted using CH2Cl2. The CH2Cl2 is then evaporated using a vacuum, creating an oil which is then dissolved in a nonaqueous ether. Finally, HCl gas is bubbled through the mixture to produce 4-methylmethcathinone hydrochloride.[61] This method produces a mixture of both enantiomers and requires similar knowledge to that required to synthesise amphetamines and MDMA.[4]: 17

It can also be produced by oxidising the ephedrine analogue 4-methylephedrine using potassium permanganate dissolved in sulfuric acid. Because 4-methylephedrine can be obtained in a specific enantiomeric form, mephedrone consisting of only one enantiomer can be produced. The danger associated with this method is it may cause manganese poisoning if the product is not correctly purified.[4]: 17
Analysis
[edit]Mephedrone does not react with most reagent testing kits. The exception is the Liebermann reagent, which gives a bright yellow reaction.[62]
History
[edit]
Mephedrone is one of hundreds of designer drugs or legal highs that have been reported in recent years, including artificial chemicals such as synthetic cannabis and semisynthetic substances such as methylhexanamine. These drugs are primarily developed to avoid being controlled by laws against illegal drugs, thus giving them the label of designer drugs.[16] According to the European Monitoring Centre for Drugs and Drug Addiction, the synthesis of mephedrone was first reported in 1929 by Saem de Burnaga Sanchez in the Bulletin de la Société Chimique de France, under the name "toluyl-alpha-monomethylaminoethylcetone",[4]: 17 [63] but the compound remained an obscure product of academia until 2003, when it was "re-discovered" and publicised by an underground chemist on The Hive website, registered to a Portuguese IP working under the pseudonym "Kinetic".[64] Kinetic posted on the site, "I've been bored over the last couple of days and had a few fun reagents lying around, so I thought I'd try and make some 1-(4-methylphenyl)-2-methylaminopropanone hydrochloride, or 4-methylmethcathinone." before going on to describe that after consuming it, the user had a "fantastic sense of well-being that I haven't got from any drug before except my beloved Ecstasy."[65] After the initial description of mephedrone's qualitative effects by Kinetic, the drug was commercially introduced in Israel by a mathematician named Ezekiel Golan or "Dr. Z".[66][67]
A drug similar to mephedrone, containing cathinone, was sold legally in Israel from around 2004, under the name hagigat. When this was made illegal, the cathinone was modified and the new products were sold by the Israeli company, Neorganics.[68][69][70] The products had names such as Neodoves pills, but the range was discontinued in January 2008 after the Israeli government made mephedrone illegal.[5][61][71] The Psychonaut Research Project, an EU organisation that searches the internet for information regarding new drugs, first identified mephedrone in 2008. Their research suggested the drug first became available to purchase on the internet in 2007, made available through British contacts, contact unknown, when it was also discussed on internet forums.[16][72] Mephedrone was first seized in France in May 2007, after police sent a tablet they assumed to be ecstasy to be analysed, with the discovery published in a paper titled "Is 4-methylephedrone, an "Ecstasy" of the twenty-first century?"[59] Mephedrone was reported as having been sold as ecstasy in the Australian city of Cairns, along with ethylcathinone, in 2008.[73][74] An annual survey of regular ecstasy users in Australia in 2010 found 21% of those surveyed had used mephedrone, with 17% having done so in the previous six months. The price they paid per gram varied from A$16 to $320.[17]
Europol noted they became aware of it in 2008, after it was found in Denmark, Finland and the UK.[75] The Drug Enforcement Administration noted it was present in the United States in July 2009.[76] By May 2010, mephedrone had been detected in all 22 EU member states that reported to Europol, as well as in Croatia and Norway.[4]: 21 The Daily Telegraph reported in April 2009 that it was manufactured in China, but it has since been made illegal there.[77][78] In March 2009, Druglink magazine reported it only cost a "couple of hundred pounds" to synthesise a kilogram of mephedrone,[68] the same month, The Daily Telegraph reported manufacturers were making "huge amounts of money" from selling it.[79] In January 2010, Druglink magazine reported dealers in Britain spent £2,500 to ship one kilogram from China, but could sell it for £10 a gram, making a profit of £7,500.[65][80] A later report, in March 2010, stated the wholesale price of mephedrone was £4000 per kilogram.[81]
In March 2011, the International Narcotics Control Board published a report about designer drugs, noting mephedrone was by then being used recreationally in Europe, North America, Southeast Asia, New Zealand and Australia.[82][83]
United Kingdom
[edit]
Between the summer of 2009 and March 2010, the use of mephedrone grew rapidly in the UK, with it becoming readily available at music festivals, head shops and on the internet.[48] A survey of Mixmag readers in 2009, found it was the fourth most popular street drug in the United Kingdom, behind cannabis, cocaine, and ecstasy.[81] The drug was used by a diverse range of social groups. Whilst the evidence was anecdotal, researchers, charity workers, teachers and users reported widespread and increasing use of the drug in 2009. The drug's rapid growth in popularity was believed to be related to both its availability and legality.[48] In a book about drugs[85] David Nutt reports the re-popularization story of mephedrone in a way that can be cross referenced with a report by Chemistry World in an article.[86]
Fiona Measham, a criminologist at the University of Lancaster, thought the emergence of mephedrone was also related to the decreasing purity of ecstasy and cocaine on sale in the UK,[48] a view reinforced in a report by the National Treatment Agency for Substance Misuse.[87] The average cocaine purity fell from 60% in 1999 to 22% in 2009 and about half of ecstasy pills seized in 2009 contained no MDMA,[88] and by June 2010 almost all ecstasy pills seized in the UK contained no MDMA.[89] A similar pattern was observed in the Netherlands, with the number of ecstasy tablets containing no MDMA rising from 10% in mid-2008 to 60% by mid-2009, with mephedrone being detected in 20% of ecstasy tablets by mid-2009.[14] The decrease of MDMA was thought to be partly due to the seizure of 33 tonnes of sassafras oil, the precursor to MDMA, in Cambodia in June 2008, which could have been used to make 245 million doses of MDMA.[65] According to John Ramsey, a toxicologist at St George's, University of London, the emergence of mephedrone was also related to the UK government banning the benzylpiperazine class of drugs in December 2009.[68][90] gamma-Butyrolactone (GBL), another previously "legal high", was also banned in August 2009 despite concerns it would be replaced by other drugs.[91]
By December 2009 mephedrone was available on at least 31 websites based in the UK and by March 2010 there were at least 78 online shops, half of which sold amounts of less than 200 grams and half that also sold bulk quantities. The price per gram varied from £9.50 to £14.[4]: 11 Between July 2009 and February 2010, UK health professionals accessed the National Poisons Information Service's (NPIS) entry on mephedrone 1664 times and made 157 telephone inquiries; the requests increased month on month over this period. In comparison, over a similar time period, the entries for cocaine and MDMA were accessed approximately 2400 times.[26] After mephedrone was made illegal the number of inquiries to the NPIS fell substantially, to only 19 in June 2010.[92]
Media organisations including the BBC and The Guardian incorrectly reported mephedrone was commonly used as a plant fertiliser. In fact sellers of the drug described it as "plant food" because it was illegal to sell the compound for human consumption.[88] In late 2009 UK newspapers began referring to the drug as meow or miaow (sometimes doubled as meow meow or miaow miaow), a name that was almost unknown on the street at the time.[93] In November 2009, the tabloid newspaper, The Sun published a story stating that a man had ripped off his own scrotum whilst using mephedrone. The story was later shown to be an online joke posted on mephedrone.com, later included in a police report with the caveat that it could be unreliable. The police report was used as a source for the story in The Sun.[46][94] Other myths the media often repeated during 2010 were that mephedrone had led to the deaths of over 20 people, teachers were unable to confiscate the drug from pupils and the government was too slow to ban the drug.[95] Parallels were drawn between the media coverage of mephedrone and a piece of satire by Chris Morris in 1997 on Brass Eye when he tricked public figures into talking of the dangers of taking the fictional legal drug "cake".[46] The Advisory Council on the Misuse of Drugs (ACMD) have suggested that the media coverage of the drug led to its increased usage.[96] Jon Silverman, a former BBC Home Affairs Correspondent, has written two articles discussing how the media had a strong influence over the UK government's drugs policy, particularly in that the government wished to demonstrate they were being "tough" on drugs.[91][97]
A survey of 1000 secondary school pupils and university students in Tayside conducted in February 2010 found 20% of them had previously taken mephedrone. Although at the time it was available legally over the internet, only 10% of users reported purchasing it online, with most purchasing it from street dealers. Of those who had used mephedrone, 97% said it was easy or very easy to obtain. Around 50% of users reported at least one negative effect associated with the use of mephedrone, of which teeth grinding is the most common.[98] Detailed interviews with users in Northern Ireland similarly found that few purchased mephedrone online, with most interviewees citing concerns that their address would be traced or that family members could intercept the package.[16]
On 30 March 2010, Alan Johnson, the then Home Secretary, announced mephedrone would be made illegal "within weeks" after the ACMD sent him a report on the use of cathinones.[9][99] The legislation would make all cathinones illegal, which Johnson said would "stop unscrupulous manufacturers and others peddling different but similarly harmful drugs".[100] The ACMD had run into problems with the UK Government in 2009 regarding drugs policy, after the government did not follow the advice of the ACMD to reclassify ecstasy and cannabis, culminating in the dismissal of the ACMD chairman, David Nutt, after he reiterated the ACMD's findings in an academic lecture.[101] Several members resigned after he was sacked, and prior to the announcement that mephedrone was to be banned, the trend continued when Dr Polly Taylor resigned, saying she "did not have trust" in the way the government would use the advice given by the ACMD.[102] Eric Carlin, a member of the ACMD and former chairman of the English Drug Education Forum, also resigned after the announcement. He said the decision by the Home Secretary was "unduly based on media and political pressure" and there was "little or no discussion about how our recommendation to classify this drug would be likely to impact on young people's behaviour."[103] Some former members of the ACMD and various charity groups expressed concern over the banning of the drug, arguing it would inevitably criminalise users, particularly young people.[104] Others expressed concern that the drug would be left in the hands of black market dealers, who will only compound the problem.[105] Carlin's resignation was specifically linked to the criminalisation of mephedrone; he stated: "We need to review our entire approach to drugs, dumping the idea that legally-sanctioned punishments for drug users should constitute a main part of the armoury in helping to solve our country's drug problems. We need to stop harming people who need help and support".[106]
The parliamentary debate was held on 8 April, one day after the 2010 general election had been announced, meaning it was during the so-called "wash-up period" when legislation is passed with little scrutiny. Only one hour was spent debating the ban and all three parties agreed, meaning no vote was required.[107] In an interview conducted in July 2010, when he was no longer a minister, Johnson admitted the decision to ban mephedrone was sped up after widespread reporting of deaths caused by the drug, and because the government wished to pass the law before parliament was dissolved prior to the upcoming general election.[91] In January 2011, however, Johnson told the Scunthorpe Telegraph that the decision was based only on information from the ACMD.[108] An editorial in the April 2010 edition of The Lancet questioned the decision to ban mephedrone, saying the ACMD did not have enough evidence to judge the potential harms caused by mephedrone and arguing that policy makers should have sought to understand why young people took it and how they could be influenced to not take it.[96] Evan Harris, then the Liberal Democrat science spokesman, stated the ACMD "was not 'legally constituted'" as required by the Misuse of Drugs Act, when the report on cathinones was published, since after Taylor resigned, it lacked a veterinary surgeon.[100] In the rush to make mephedrone illegal, the act that was passed specified the inactive enantiomer of mephedrone, leaving the active form legal until the loophole was closed in February 2011 by another act of parliament.[109] In Chemistry World, John Mann, professor of chemistry at Queen's University Belfast, suggested the UK create a law similar to the Federal Analog Act of the United States, which would have made mephedrone illegal as an analog of cathinone.[110] In August 2010, James Brokenshire, the Home Office drugs minister, announced plans to create a new category in the Misuse of Drugs Act, through the Police Reform and Social Responsibility Bill, that would allow new legal highs to be made temporarily illegal, without the need for a vote in parliament or advice from the ACMD, as was required to categorise mephedrone.[111][112][113]
According to the Independent Scientific Committee on Drugs, after mephedrone was made illegal, a street trade in the drug emerged, with prices around double those prior to the ban, at £20–£25 per gram.[114] In September 2010, Druglink reported the ban had a mixed effect on mephedrone use, with it decreasing in some areas, remaining similar in others and becoming more prevalent in some areas.[115] In an online survey of 150 users after the ban, 63% said they were continuing to use mephedrone; of those, half claimed unchanged usage amounts (as to dosage and frequency) and half claimed decreased usage. Compared to previous surveys, more users purchased it from dealers, rather than the internet. The average price per gram was £16, compared to around £10 before the ban.[116] The 2010 Mixmag survey of 2,500 nightclubbers found one quarter had used mephedrone in the previous month, the price had roughly doubled since it was made illegal, and it was more likely to be cut with other substances.[117] Of those who had already used mephedrone prior to the ban, 75% had continued to use it after the ban. Of the various drugs used by the survey participants, users were more likely to have concerns about it.[118] Interviews with users in Northern Ireland also found the price had roughly doubled since it was made illegal, to around £30 a gram. Rather than the price rising due to increased scarcity of the drug, it is thought to have risen for two other reasons. Firstly, dealers knew there was still demand for mephedrone, but were aware the supplies may be exhausted in the future. Secondly, the dealers perceived customers were likely to be willing to pay more for an illegal substance.[16]
Professor Shiela Bird, a statistician at the Medical Research Council, suggested the ban of mephedrone may lead to more cocaine-related deaths. In the first six months of 2009, the number of cocaine-related deaths fell for the first time in four years, and fewer soldiers tested positive for cocaine in 2009 than in 2008. She suggested this may have been due to users switching to mephedrone from cocaine, but cautioned that before full figures are available for 2009 and 2010, it will be difficult to determine whether mephedrone saved lives, rather than cost them.[119][120] Other supposedly legal drugs have filled the gap in the market since mephedrone was made illegal, including naphyrone (NRG-1) (since made illegal)[121] and Ivory Wave, which has been found to contain MDPV, a compound made illegal at the same time as mephedrone. However, some products branded as Ivory Wave possibly do not contain MDPV.[122] When tested, some products sold six weeks after mephedrone was banned, advertised as NRG-1, NRG-2 and MDAI, were found to be mephedrone.[123] A Drugscope survey of drugs workers at the end of 2012 reported that mephedrone use was still widespread in the UK and that there increasing reports of problematic users. It was being taken as not only a "poor man's cocaine" but also amongst users of heroin and crack cocaine. Cases of intravenous use were also reported to be on the increase.[124]
Society and culture
[edit]Legal status
[edit]
When mephedrone was rediscovered in 2003, it was not specifically illegal to possess in any country. As its use has increased, many countries have passed legislation making its possession, sale and manufacture illegal. It was first made illegal in Israel, where it had been found in products such as Neodoves pills, in January 2008.[5] After the death of a young woman in Sweden in December 2008 was linked to the use of mephedrone, it was classified as a hazardous substance a few days later, making it illegal to sell in Sweden. In June 2009, it was classified as a narcotic with the possession of 15 grams or more resulting in a minimum of two years in prison—a longer sentence, gram for gram than given for the possession of cocaine or heroin.[125][126] In December 2008, Denmark also made it illegal[127] and through the Medicines Act of Finland, it was made illegal to possess without a prescription.[128] In November 2009, it was classified as a "narcotic or psychotropic" substance and added to the list of controlled substances in Estonia[129] and made illegal to import into Guernsey along with other legal highs,[130] before being classified as a Class B drug in April 2010.[131] It was classified as a Class C drug in Jersey in December 2009.[132]
In 2010, as its use became more prevalent, many countries passed legislation prohibiting mephedrone. It became illegal in Croatia[133] and Germany[134] in January, followed by Romania[135] and the Isle of Man in February.[136] In March 2010, it was classified as an unregulated medicine in the Netherlands, making the sale and distribution of it illegal.[137][138] The importation of mephedrone into the UK was banned on 29 March 2010.[139] The next day, the ACMD in the UK published a report on the cathinones, including mephedrone, and recommended they be classified as Class B drugs. On 7 April 2010, the Misuse of Drugs Act 1971 (Amendment) Order 2010 was passed by parliament, making mephedrone and other substituted cathinones, Class B drugs from 16 April 2010.[140][141] Prior to the ban taking effect, mephedrone was not covered by the Misuse of Drugs Act 1971.[77] It was, though, an offence under the Medicines Act to sell it for human consumption, so it was often sold as "plant food" or "bath salts", although it has no use as these products; this, too, was possibly illegal under the Trade Descriptions Act 1968.[24][25][9] In the US, similar descriptions have been used to describe mephedrone, as well as methylenedioxypyrovalerone (MDPV).[142] In May 2010, the Republic of Ireland made mephedrone illegal,[143][144][145] followed by Belgium,[146] Italy,[147] Lithuania,[148] France[149][150] and Norway[151] in June and Russia in July.[152] In August 2010, Austria[153] and Poland[154] made it illegal and China announced it would be illegal as of 1 September 2010.[78] Mephedrone had been reported to be used in Singapore in February 2010,[155] but it was made illegal in November 2010.[156] In December 2010, following the advice of the EMCDDA, mephedrone was made illegal throughout the EU, a move Switzerland also made shortly afterwards.[157][158] Countries which have not already banned it, such as the Netherlands, Greece and Portugal, will need to change legislation to comply with the EU ruling.[158] In Hungary, a government advisory body recommended mephedrone should be made illegal in August 2010, which was followed, making it illegal in January 2011;[159][160] Spain followed in February 2011.[161] Mexico, by decree,[162] outlawed mephedrone as a substance "with low or no therapeutical use which pose a serious threat to public health"[163] in 2014.
In some countries, mephedrone is not specifically listed as illegal, but is controlled under legislation that makes compounds illegal if they are analogs of drugs already listed. In Australia during 2010, it was not specifically listed as prohibited,[61] but the Australian Federal Police stated it is an analogue to methcathinone and therefore illegal. It is now listed as a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015).[164] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[164] In February 2010, 22 men were arrested in connection with importing mephedrone.[165] By January 2011, every state in Australia, other than Victoria, had listed it as a controlled drug.[166]
In New Zealand, it is not included in the Misuse of Drugs Act 1975,[167] but is illegal, as it is similar to controlled substances.[168]
In Canada, mephedrone is not explicitly listed in any schedule of the Controlled Drugs and Substances Act, but "amphetamines, their salts, derivatives, isomers and analogues and salts of derivatives, isomers and analogues" are included in Section 19 of Schedule I of the act. Cathinone and methcathinone are listed in separate sections of Schedule III, while diethylpropion and pyrovalerone (also cathinones), are listed in separate sections of Schedule IV, each without language to capture analogues, isomers, etc.[169] Mephedrone is considered a controlled substance by Health Canada.[170] In a report by the Canadian Medical Association in 2010, one lawyer was quoted as suggesting that mephedrone was less popular in Canada than in the U.K. because "there's a provision in the substance act that says analogues of certain drugs and other similar drugs may be illegal too,"; on the other hand, the assistant director of the Centre for Addictions Research of British Columbia suggested there was a lack of "clear illegality".[171] There have been several media reports of the Canadian police seizing mephedrone,[172][173][174] but no reports of any successful prosecution of a Controlled Drugs and Substances Act offence implicating mephedrone.
Mephedrone is also currently scheduled in the United States as of 2011. The Drug Enforcement Administration (DEA) states, as an analogue of methcathinone, possession of mephedrone can be controlled by the Federal Analog Act, but according to the Los Angeles Times, this only applies if it is sold for human consumption.[175][176][177] Several cities and states, such as New York state,[178] have passed legislation to specifically list mephedrone as illegal, but in most areas it remained legal, so long as it is not sold for human consumption, so retailers described it as 'bath salts'.[177] In September 2011, The DEA began using its emergency scheduling authority to temporarily control mephedrone. Except as authorised by law, this action made possessing and selling mephedrone or the products that contain it illegal in the US for at least one year while the DEA and the United States Department of Health and Human Services conduct further study.[179] Control of these compounds became permanent on 9 July 2012, via passage of the Synthetic Drug Abuse Prevention Act of 2012.[180]
Usage
[edit]A survey conducted in late 2009 by the National Addiction Centre (UK) found 41.3% of readers of Mixmag had used mephedrone in the last month, making it the fourth-most popular drug amongst clubbers. Of those, two-thirds snorted the drug and the average dosage per session was 0.9 g; the length of sessions increased as the dosage increased. Users who snorted the drug reported using more per session than those who took it orally (0.97 g compared to 0.74 g) and also reported using it more often (five days per month compared to three days per month).[15] An Irish study of people on a methadone treatment program for heroin addicts found 29 of 209 patients tested positive for mephedrone usage.[181]
Harm assessment
[edit]Professor David Nutt, former chair of the Advisory Council on the Misuse of Drugs (ACMD) in the UK has said in 2009, "people are better off taking ecstasy or amphetamines than those [drugs] we know nothing about" and "Who knows what's in [mephedrone] when you buy it? We don't have a testing system. It could be very dangerous, we just don't know. These chemicals have never been put into animals, let alone humans."[182] Les King, a former member of the ACMD, has stated mephedrone appears to be less potent than amphetamine and ecstasy, but that any benefit associated with this could be negated by users taking larger amounts. He also told the BBC, "all we can say is [mephedrone] is probably as harmful as ecstasy and amphetamines and wait until we have some better scientific evidence to support that."[58]
See also
[edit]Notes
[edit]References
[edit]- ^ Meyer MR, Peters FT, Maurer HH (2009). "Metabolism of the new designer drug mephedrone and toxicological detection of the beta keto designer drugs mephedrone, butylone and methylone in urine" (PDF). Primary. Annales de Toxicologie Analytique. 21 (S1): 22.
- ^ Anvisa (24 July 2023). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 25 July 2023). Archived from the original on 27 August 2023. Retrieved 27 August 2023.
- ^ "Substance Details 4-Methylmethcathinone". Retrieved 22 January 2024.
- ^ a b c d e f g h i j k l m n o p "Europol–EMCDDA Joint Report on a new psychoactive substance: 4-methylmethcathinone (mephedrone)". European Monitoring Centre for Drugs and Drug Addiction. 27 May 2010. Archived from the original (PDF) on 26 January 2012. Retrieved 29 January 2011.
- ^ a b c d Cumming E (22 April 2010). "Mephedrone: Chemistry lessons". The Daily Telegraph. London. Archived from the original on 12 January 2022. Retrieved 14 September 2010.
- ^ "Drugs crackdown hailed a success". BBC News. 8 March 2010. Retrieved 31 March 2010.
- ^ Kihara R, Day E (May 2014). "Transient psychotic episodes following recreational use of NRG-3". Primary. Progress in Neurology and Psychiatry. 18 (3): 14–18. doi:10.1002/pnp.331. S2CID 70766780.
- ^ Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, et al. (April 2011). "Mephedrone (4-methylmethcathinone; 'meow meow'): chemical, pharmacological and clinical issues" (PDF). Review. Psychopharmacology. 214 (3): 593–602. doi:10.1007/s00213-010-2070-x. hdl:2299/16594. PMID 21072502. S2CID 10529974.
- ^ a b c "Consideration of the cathinones". Advisory Council on the Misuse of Drugs. 31 March 2010. p. 25. Archived from the original on 8 December 2010. Retrieved 10 February 2011.
- ^ "Mephedrone". Alcohol and Drug Foundation. 10 November 2021. Archived from the original on 18 April 2021. Retrieved 22 November 2021.
- ^ a b c d e f g h i j k l m Papaseit E, Moltó J, Muga R, Torrens M, de la Torre R, Farré M (2017). "Clinical Pharmacology of the Synthetic Cathinone Mephedrone". Review. Current Topics in Behavioral Neurosciences. 32: 313–331. doi:10.1007/7854_2016_61. ISBN 978-3-319-52442-9. PMID 28012094.
- ^ a b White CM (November 2016). "Mephedrone and 3,4-Methylenedioxypyrovalerone (MDPV): Synthetic Cathinones With Serious Health Implications". Review. Journal of Clinical Pharmacology. 56 (11): 1319–1325. doi:10.1002/jcph.742. PMID 27029951.
- ^ "Synthetic Drug Abuse Prevention Act (SDAPA)" (PDF). United States Congress.
- ^ a b c d Brunt TM, Poortman A, Niesink RJ, van den Brink W (November 2011). "Instability of the ecstasy market and a new kid on the block: mephedrone". Review. Journal of Psychopharmacology. 25 (11): 1543–7. doi:10.1177/0269881110378370. PMID 20826554. S2CID 7207426.(subscription required)
- ^ a b c Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F (January 2011). "Mephedrone, new kid for the chop?". Primary. Addiction. 106 (1): 154–61. doi:10.1111/j.1360-0443.2010.03130.x. PMID 20735367.
- ^ a b c d e McElrath K, O'Neill C (March 2011). "Experiences with mephedrone pre- and post-legislative controls: perceptions of safety and sources of supply". Primary. The International Journal on Drug Policy. 22 (2): 120–7. doi:10.1016/j.drugpo.2010.11.001. PMID 21242082.(subscription required)
- ^ a b Matthews AJ, Bruno RB (2010). "Mephedrone use among regular ecstasy consumers in Australia" (PDF). Ecstasy and Related Drug Trends Bulletin. Archived from the original (PDF) on 17 February 2011.
- ^ "A joined-up approach to drugs is needed now". Herald Scotland. 21 March 2010. Retrieved 15 September 2010.
- ^ a b Gibbons S, Zloh M (July 2010). "An analysis of the 'legal high' mephedrone". Primary. Bioorganic & Medicinal Chemistry Letters. 20 (14): 4135–9. doi:10.1016/j.bmcl.2010.05.065. PMID 20542690.(subscription required)
- ^ "Risks of banned drug mephedrone revealed in new research". Science Daily. 15 July 2010. Retrieved 10 February 2011.
- ^ Kavanagh PV, McNamara S, Angelov D, McDermott S, Mullan D, Ryder SA (March 2010). "The Characterization of 'Legal Highs' Available from Head Shops in Dublin" (PDF). The Drug Treatment Centre Board. Retrieved 17 February 2011.
- ^ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Primary. Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- ^ Winstock AR, Marsden J, Mitcheson L (March 2010). "What should be done about mephedrone?". BMJ. 340: c1605. doi:10.1136/bmj.c1605. PMID 20332508. S2CID 5500659.
- ^ a b "Police warning over 'bubble' drug". BBC News. 20 November 2009. Retrieved 27 November 2009.
- ^ a b Reed J (13 January 2010). "Clubbers are 'turning to new legal high mephedrone'". BBC News. Retrieved 4 July 2010.
- ^ a b James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, et al. (National Poisons Information Service) (August 2011). "Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service". Primary. Emergency Medicine Journal. 28 (8): 686–9. doi:10.1136/emj.2010.096636. PMC 3143586. PMID 20798084.
- ^ Wood DM, Greene SL, Dargan PI (April 2011). "Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone". Primary. Emergency Medicine Journal. 28 (4): 280–2. doi:10.1136/emj.2010.092288. PMID 20581379. S2CID 26029573.(subscription required)
- ^ a b Daziani G, Lo Faro AF, Montana V, Goteri G, Pesaresi M, Bambagiotti G, et al. (March 2023). "Synthetic Cathinones and Neurotoxicity Risks: A Systematic Review". Int J Mol Sci. 24 (7): 6230. doi:10.3390/ijms24076230. PMC 10093970. PMID 37047201.
- ^ Karch SB (January 2015). "Cathinone neurotoxicity (The "3Ms")". Review. Current Neuropharmacology. 13 (1): 21–5. doi:10.2174/1570159X13666141210225009. PMC 4462040. PMID 26074741.
- ^ a b Pantano F, Tittarelli R, Mannocchi G, Pacifici R, di Luca A, Busardò FP, et al. (2017). "Neurotoxicity Induced by Mephedrone: An up-to-date Review". Review. Current Neuropharmacology. 15 (5): 738–749. doi:10.2174/1570159X14666161130130718. PMC 5771050. PMID 27908258.
- ^ Mead J, Parrott A (May 2020). "Mephedrone and MDMA: A comparative review". Review. Brain Res. 1735: 146740. doi:10.1016/j.brainres.2020.146740. PMID 32087112.
- ^ Kamińska K, Noworyta-Sokołowska K, Górska A, Rzemieniec J, Wnuk A, Wojtas A, et al. (October 2018). "The Effects of Exposure to Mephedrone During Adolescence on Brain Neurotransmission and Neurotoxicity in Adult Rats". Review. Neurotox Res. 34 (3): 525–537. doi:10.1007/s12640-018-9908-0. PMC 6154178. PMID 29713996.
- ^ López-Arnau R, Camarasa J, Carbó ML, Nadal-Gratacós N, Puigseslloses P, Espinosa-Velasco M, et al. (2022). "3,4-Methylenedioxy methamphetamine, synthetic cathinones and psychedelics: From recreational to novel psychotherapeutic drugs". Front Psychiatry. 13: 990405. doi:10.3389/fpsyt.2022.990405. PMC 9574023. PMID 36262632.
- ^ Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. (April 2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–1203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H, et al. (September 2010). "Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity". Primary. Journal of Medical Toxicology. 6 (3): 327–30. doi:10.1007/s13181-010-0018-5. PMC 3550484. PMID 20358417.(subscription required)
- ^ Garrett G, Sweeney M (September 2010). "The serotonin syndrome as a result of mephedrone toxicity". Primary. BMJ Case Reports. 2010: bcr0420102925. doi:10.1136/bcr.04.2010.2925. PMC 3029518. PMID 22778288.(subscription required)
- ^ Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O'Riordan JI (August 2010). "A harmless high?". Primary. Lancet. 376 (9742): 742. doi:10.1016/S0140-6736(10)60891-4. PMID 20801405. S2CID 205959146.(subscription required)
- ^ Nicholson PJ, Quinn MJ, Dodd JD (December 2010). "Headshop heartache: acute mephedrone 'meow' myocarditis". Primary. Heart. 96 (24): 2051–2. doi:10.1136/hrt.2010.209338. PMID 21062771. S2CID 36684597.(subscription required)
- ^ Ahmed N, Hoy BP, McInerney J (October 2010). "Methaemoglobinaemia due to mephedrone ('snow')". Primary. BMJ Case Reports. 2010: bcr0420102879. doi:10.1136/bcr.04.2010.2879. PMC 3027398. PMID 22791577.(subscription required)
- ^ a b "Teenager dies of 'net drug' overdose". The Local. 15 December 2008. Retrieved 20 September 2010.
- ^ Gustavsson D, Escher C (20 October 2009). "Mefedron – Internetdrog som tycks ha kommit för att stanna" [Mephedrone – Internet drug that seems to have come to stay]. Läkartidningen (in Swedish). Archived from the original on 22 October 2009. Retrieved 21 February 2010.
- ^ Ghodse H, Corkery J, Ahmed K, Naidoo V, Oyefeso A, Schifano F (July 2010). "Drug-related deaths in the UK: Annual Report 2010" (PDF). International Centre for Drug Policy, St George's University of London. p. 77. Archived from the original (PDF) on 29 December 2010. Retrieved 2 January 2010.
- ^ "Man from Hove died after injecting mephedrone". BBC News. 27 May 2010. Retrieved 9 June 2012.
- ^ a b "Teenagers' deaths 'not caused by mephedrone'". BBC News. 28 May 2010. Retrieved 20 September 2010.
- ^ Torrance H, Cooper G (October 2010). "The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland". Primary. Forensic Science International. 202 (1–3): e62-3. doi:10.1016/j.forsciint.2010.07.014. PMID 20685050.
- ^ a b c Fleming N (5 April 2010). "Mephedrone: the anatomy of a media drug scare". The Guardian. London. Retrieved 20 September 2010.
- ^ "Scunthorpe community 'awash with methadone'". BBC News. 25 January 2011. Retrieved 10 February 2011.
- ^ a b c d Measham F, Moore K, Newcombe R, Welch Z (12 March 2010). "Tweaking, bombing, dabbing and stockpiling: the emergence of mephedrone and the perversity of prohibition". Drugs and Alcohol Today. 10 (1): 14–21. doi:10.5042/daat.2010.0123. S2CID 56998007.(subscription required)
- ^ Dickson AJ, Vorce SP, Levine B, Past MR (April 2010). "Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin". Primary. Journal of Analytical Toxicology. 34 (3): 162–8. doi:10.1093/jat/34.3.162. PMID 20406541.(subscription required)
- ^ Darke S, Zador D (December 1996). "Fatal heroin 'overdose': a review". Review. Addiction. 91 (12): 1765–72. doi:10.1046/j.1360-0443.1996.911217652.x. PMID 8997759. Archived from the original on 23 December 2002. Retrieved 13 September 2010.
- ^ a b c d Czerwinska J, Parkin MC, George C, Kicman AT, Dargan PI, Abbate V (August 2021). "Pharmacokinetics of Mephedrone and Its Metabolites in Whole Blood and Plasma after Controlled Intranasal Administration to Healthy Human Volunteers". primary. Journal of Analytical Toxicology. 45 (7): 730–738. doi:10.1093/jat/bkaa134. PMID 32986113.
- ^ Czerwinska J, Parkin MC, Cilibrizzi A, George C, Kicman AT, Dargan PI, et al. (December 2020). "Pharmacokinetics of Mephedrone Enantiomers in Whole Blood after a Controlled Intranasal Administration to Healthy Human Volunteers". Primary. Pharmaceuticals. 14 (1). Basel, Switzerland: 5. doi:10.3390/ph14010005. PMC 7822411. PMID 33374623.
- ^ Mayer FP, Wimmer L, Dillon-Carter O, Partilla JS, Burchardt NV, Mihovilovic MD, et al. (September 2016). "Phase I metabolites of mephedrone display biological activity as substrates at monoamine transporters". Primary. British Journal of Pharmacology. 173 (17): 2657–68. doi:10.1111/bph.13547. PMC 4978154. PMID 27391165.
- ^ Olesti E, Farré M, Papaseit E, Krotonoulas A, Pujadas M, de la Torre R, et al. (November 2017). "Pharmacokinetics of Mephedrone and Its Metabolites in Human by LC-MS/MS". Primary. The AAPS Journal. 19 (6): 1767–1778. doi:10.1208/s12248-017-0132-2. PMID 28828691.
- ^ a b Meyer MR, Wilhelm J, Peters FT, Maurer HH (June 2010). "Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry". Primary. Analytical and Bioanalytical Chemistry. 397 (3): 1225–33. doi:10.1007/s00216-010-3636-5. PMID 20333362. S2CID 21471611.(subscription required)
- ^ Marinetti LJ, Antonides HM (April 2013). "Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results". Review. Journal of Analytical Toxicology. 37 (3): 135–46. doi:10.1093/jat/bks136. PMID 23361867.
- ^ Baselt RC (2014). Disposition of toxic drugs and chemicals in man (10th ed.). Seal Beach, California: Biomedical Publications. pp. 1226–1227. ISBN 978-0-9626523-9-4.
- ^ a b Reed J (13 January 2010). "What is legal high mephedrone?". BBC Newsbeat. Retrieved 20 September 2010.
- ^ a b Roussel O, Perrin M, Herard P, Chevance M, Arpino P (2009). "La 4-méthyléphédrone sera-t-elle une "Ecstasy" du XXIème siècle" [Is 4-methylephedrone, an "Ecstasy" of the twenty first century?] (PDF). Annales de Toxicologie Analytique (in French). doi:10.1051/ata/2009048.
- ^ Psychonaut WebMapping Research Group (March 2010). "Mephedrone Report" (PDF). Institute of Psychiatry, King's College London. Archived from the original (PDF) on 4 July 2011. Retrieved 31 January 2011.
- ^ a b c Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG (April 2010). "Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone". Primary. Forensic Science International. 197 (1–3): 59–66. doi:10.1016/j.forsciint.2009.12.048. PMID 20074881.(subscription required)
- ^ Toole KE, Fu S, Shimmon RG, Kraymen N (2012). "Color Tests for the Preliminary Identification of Methcathinone and Analogues of Methcathinone" (PDF). Primary. Microgram Journal. 9 (1): 27–32. Archived from the original (PDF) on 26 November 2013. Retrieved 29 January 2016.
- ^ Saem de Burnaga Sanchez J (1929). "Sur un homologue de l'éphédrine" [On an analogue of ephedrine]. Bulletin de la Société Chimique de France (in French). 45: 284–286.
- ^ Morris H (5 April 2010). "Hamilton's Pharmacopeia. Mephedrone: the phantom menace". Vice Magazine. Retrieved 4 July 2010.
- ^ a b c Power M (January–February 2010). "How mephedrone shook the drug world – World Wired Web" (PDF). Druglink. Drugscope. pp. 11–13. Archived from the original (PDF) on 21 August 2010. Retrieved 16 September 2010.
- ^ Slezak M (10 March 2014). "High as a Kiwi: Inside the nation saying yes to drugs". New Scientist.
- ^ Slezak M (11 August 2014). "My recreational drugs can defeat organised crime". New Scientist.
- ^ a b c Power M (March–April 2009). "Mephedrone: the future of drug dealing?" (PDF). Druglink. Drugscope. pp. 6–9. Archived from the original (PDF) on 21 August 2010. Retrieved 16 September 2010.
- ^ Doward J, Shah O (26 April 2009). "There are many drugs that help people get out of their minds yet stay within the law – they're called 'legal highs'". The Observer. Retrieved 28 October 2010.
- ^ Bentur Y, Bloom-Krasik A, Raikhlin-Eisenkraft B (March 2008). "Illicit cathinone ("Hagigat") poisoning". Primary. Clinical Toxicology. 46 (3): 206–10. doi:10.1080/15563650701517574. PMID 17852166. S2CID 23603259.(subscription required)
- ^ Davies S, Ramsey J, Archer R (November 2009). "Analytical profiles of Methcathinone Related Compounds" (PDF). London Toxicology Group. Retrieved 22 March 2010.
- ^ "Psychonaut Web Mapping Project Newsletter". Psychonaut Web Mapping Project. June–September 2009. Archived from the original on 16 February 2011. Retrieved 19 December 2009.
- ^ Guppy D (18 June 2008). "Killer pills hit Cairns". Cairns.com.au. Retrieved 11 August 2009.
- ^ "Police warn of potentially fatal 'fake ecstasy'". Abc.net.au. 17 June 2008. Archived from the original on 10 February 2010. Retrieved 11 August 2009.
- ^ "EMCDDA 2008 Annual Report" (PDF). European Monitoring Centre for Drugs and Drug Addiction. Retrieved 26 November 2009.
- ^ "DEA Microgram Bulletin – 4-Methylmethcathinone in Oregon" (PDF). The Drug Enforcement Administration. July 2009. Archived from the original (PDF) on 17 October 2010. Retrieved 29 January 2011.
- ^ a b Devlin K (30 April 2009). "Psychiatrists call for 'legal high' drug 4-MMC to be banned". The Daily Telegraph. London. Archived from the original on 26 July 2009. Retrieved 16 September 2010.
- ^ a b "Mephedrone regulated as psychotropic substances". State Food and Drug Administration, P.R. China. 2 August 2010. Archived from the original on 15 August 2010. Retrieved 12 September 2010.
- ^ Gammell C (12 March 2009). "Legal online drugs providing real alternative to Class A substances". The Daily Telegraph. London. Archived from the original on 12 September 2009. Retrieved 16 September 2010.
- ^ Campbell D (17 January 2010). "Fears grow over safety of 'legal high' mephedrone". London: The Observer. Retrieved 19 March 2010.
- ^ a b Fleming N (18 March 2010). "Briefing: Should miaow-miaow be banned?". New Scientist. Retrieved 16 September 2010.
- ^ "Designer drug abuse out of control: U.N. drugs board; abuse of 4-methyl-methcathinone, or mephedrone". Reuters. 2 March 2011. Archived from the original on 30 July 2012. Retrieved 26 March 2012.
- ^ INCB Annual Report 2010 (PDF) (Report). International Narcotics Control Board. March 2011. Archived from the original (PDF) on 2 April 2012. Retrieved 26 March 2012.
- ^ Dargan P, Wood D (July 2010). Annex 1 to the Risk assessment report: Technical report on mephedrone (PDF) (Report). European Monitoring Centre for Drugs and Drug Addiction. Archived from the original (PDF) on 15 July 2011. Retrieved 24 September 2010.
- ^ Nutt DJ (2021). Drugs without the hot air : making sense of legal and illegal drugs (Second ed.). Cambridge: Bloomsbury Publishing. ISBN 978-0-85784-498-9. Archived from the original on 27 September 2022. Retrieved 27 September 2022.
- ^ Extance A. "The rising tide of 'legal highs'". Chemistry World. Retrieved 27 September 2022.
- ^ Drug Treatment in 2009–10 (PDF) (Report). National Treatment Agency for Substance Misuse. October 2010. Archived from the original (PDF) on 1 March 2011. Retrieved 27 October 2010.
- ^ a b Fleming N (29 March 2010). "Miaow-miaow on trial: Truth or trumped-up charges?". New Scientist. Retrieved 1 April 2010.
- ^ Reed J (20 June 2010). "Newsbeat – Ecstasy 'disappearing' from British clubs". BBC. Retrieved 30 August 2010.
- ^ "The Misuse of Drugs Act 1971 (Amendment) Order 2009". UK government. October 2009. Retrieved 31 January 2011.
- ^ a b c Silverman J (2010). "Addicted to distortion: the media and UK drugs policy". Safer Communities. 9 (4): 26–31. doi:10.5042/sc.2010.0582.(subscription required)
- ^ "Inquiries over mephedrone effects". Press Association. 4 October 2010. Archived from the original on 15 July 2011. Retrieved 27 October 2010.
- ^ Private Eye, "Street of Shame", No. 1259, 2–15 April 2010, p. 6: Way back in January 2009, not long after mephedrone first began to be sold online, members of the web forum attached to the now-defunct "headshop" Champagne Legals discussed what brand name they might attach to the new product, which has the chemical identity dimethylmethcathinone or MM-Cat. "What shall we call this drug? It's called MM-CAT, so why not Miaow?" suggested one. The name did not catch on ... But on 1 November 2009, someone did add the name "Meow" to the Wikipedia entry for Mephedrone at the head of a list of "street names." Three weeks later ... the Sun declared the arrival of a "new party favourite called 'meow meow'" and the world went cat-call crazy. Among a host of recent headlines the Sunday Times has reported on "the rise of Meow", the Times has heralded "Meow Meow arrests", the Sun shrieked about a "Harman snub for Meow Meow Ban" and the Daily Telegraph took a long hard look at the "Meow Meow Menace in Europe." "No one ever called it Meow seriously till the papers picked up on the Wikipedia entry," one drugs expert tells the Eye.
- ^ Davey Z, Corazza O, Schifano F, Deluca P (2010). "Mass-information: mephedrone, myths, and the new generation of legal highs". Review. Drugs and Alcohol Today. 10 (3): 24–28. doi:10.5042/daat.2010.0467.(subscription required)
- ^ Greenslade R (2 December 2010). "New guide aims to bring sense to the media coverage of drugs". The Guardian. Retrieved 19 December 2010.
- ^ a b Anonymous editorial (April 2010). "A collapse in integrity of scientific advice in the UK". Lancet. 375 (9723): 1319. doi:10.1016/S0140-6736(10)60556-9. PMID 20399965. S2CID 39998854.
- ^ Silverman J (2010). "Addicted to getting drugs wrong". British Journalism Review. 21 (4): 31–36. doi:10.1177/0956474810393603. S2CID 143188880. Archived from the original on 12 August 2011.
- ^ Dargan PI, Albert S, Wood DM (November 2010). "Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change". Primary. QJM. 103 (11): 875–9. doi:10.1093/qjmed/hcq134. PMID 20675396.
- ^ "Mephedrone to be made Class B drug 'within weeks'". BBC News. 29 March 2010. Retrieved 31 March 2010.
- ^ a b Kmietowicz Z (March 2010). "Home secretary bans mephedrone after taking advice from depleted council". BMJ. 340: c1784. doi:10.1136/bmj.c1784. PMID 20356967. S2CID 8396924.
- ^ Taylor P (29 March 2010). "Drug adviser Dr Polly Taylor's full resignation letter". BBC News. Retrieved 15 September 2010.
- ^ "Resignation 'threatens drug ban'". BBC News. 29 March 2010. Retrieved 29 March 2010.
- ^ "Government adviser Eric Carlin resigns over mephedrone". BBC News. 2 April 2010. Retrieved 2 April 2010.
- ^ Rolles S (18 March 2010). "Mephredone and the ACMD: lessons from BZP and New Zealand's "Class D' experiment?". Transform Drug Policy Foundation. Archived from the original on 15 October 2010. Retrieved 13 April 2010.
- ^ Doward J (4 April 2010). "Mephedrone row grows as seventh member of drugs panel". London: The Observer. Retrieved 15 September 2010.
- ^ Carlin E (2 April 2010). "Eric Carlin's letter of resignation from the ACMD". BBC News. Retrieved 13 April 2010.
- ^ Eastwood N (June 2010). "Legal Eye – Mephedrone becomes a class B drug". Drugs and Alcohol Today. 10 (2): 6–9. doi:10.5042/daat.2010.0251.(subscription required)
- ^ "Decision to outlaw mephedrone drug not connected to teen deaths". The Scunthorpe Telegraph. 27 January 2011. Archived from the original on 8 March 2012. Retrieved 10 February 2011.
- ^ Nutt D (April 2011). "Perverse effects of the precautionary principle: how banning mephedrone has unexpected implications for pharmaceutical discovery". Primary. Therapeutic Advances in Psychopharmacology. 1 (2): 35–6. doi:10.1177/2045125311406958. PMC 3736901. PMID 23983925.
- ^ Mann J (April 2010). "Can we halt the flow of new designer drugs?". Chemistry World. Retrieved 16 September 2010.
- ^ Sare J (September–October 2010). "An unchartered course" (PDF). Druglink. Drugscope. p. 5. Archived from the original (PDF) on 27 September 2011. Retrieved 29 January 2011.
- ^ Stratton E (8 December 2010). "Proposals for banning drugs are more draconian than they seem". The Guardian. Retrieved 19 December 2010.
- ^ Van Noorden R (6 December 2010). "Science advice not mandatory on drugs council, proposes UK government". Nature News. Archived from the original on 28 December 2010. Retrieved 20 December 2010.
- ^ "Mephedrone: harm reduction advice". Drugscience.org.uk. Independent Scientific Committee on Drugs. 2010. Archived from the original on 2 July 2012. Retrieved 15 September 2010.
- ^ Daly M (September–October 2010). "Booze, bans and bite-size bags" (PDF). Druglink. Drugscope. pp. 6–9. Archived from the original (PDF) on 27 September 2011. Retrieved 29 January 2011.
- ^ Winstock A, Mitcheson L, Marsden J (November 2010). "Mephedrone: still available and twice the price". Lancet. 376 (9752): 1537. doi:10.1016/S0140-6736(10)62021-1. PMID 21056754. S2CID 41708575.
- ^ "Clubbers 'still using' banned drug mephedrone". BBC News. 8 February 2011. Retrieved 10 February 2011.
- ^ Garnett N (9 February 2011). "Mephedrone freely available on the internet despite ban". BBC Radio 5 Live. Retrieved 17 February 2011.
- ^ Laurance J (24 November 2010). "Mephedrone ban blamed for rise in cocaine deaths". The Independent. Archived from the original on 12 May 2022. Retrieved 20 December 2010.
- ^ Bird S (22 November 2010). "Banned drug may have saved lives, not cost them". Straight Statistics. Retrieved 20 December 2010.
- ^ "NRG-1 'legal high' drug is banned". BBC News. 12 July 2010. Retrieved 23 August 2010.
- ^ Jones S, Power M (17 August 2010). "Ivory Wave drug implicated in death of 24-year-old man". The Guardian. London. Retrieved 23 August 2010.
- ^ Brandt SD, Sumnall HR, Measham F, Cole J (July 2010). "Second generation mephedrone. The confusing case of NRG-1". Primary. BMJ. 341: c3564. doi:10.1136/bmj.c3564. PMID 20605894. S2CID 20354123.(subscription required)
- ^ Daly M (December 2012). "'Drone Strikes" (PDF). Druglink. Drugscope. pp. 8–11. Retrieved 28 March 2013.
- ^ "Svensk författningssamling Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor" [Swedish Code of Statutes. Regulation amending the Ordinance (1999:58) banning certain hazardous goods] (PDF) (in Swedish). 25 November 2008. Archived from the original (PDF) on 5 July 2011. Retrieved 31 January 2011.
- ^ Stigson G (9 June 2010). "15 gram narkotika ger två års fängelse" [15 grams of drugs means two years in prison] (in Swedish). Dala Demokraten. Archived from the original on 19 July 2011. Retrieved 20 September 2010.
- ^ 2009 National Report (2008 data) To the EMCDDA by the Reitox National Focal Point – Denmark (PDF) (Report). 2009. Archived from the original (PDF) on 15 July 2011. Retrieved 14 September 2010.
- ^ "Rapujuhlat saaristossa" [Crayfish parties in the archipelago] (in Finnish). City Magazine. September 2009. Retrieved 14 September 2010.
- ^ Estonian Ministry of Social Affairs (8 December 2009). "Sotsiaalministri 27.11.2009 määrus number 87" [Minister of Social Affairs regulation number 87] (in Estonian). Archived from the original on 20 July 2011. Retrieved 21 September 2010.
- ^ "Guernsey mephedrone ban 'only weeks away'". BBC News. 6 April 2010. Retrieved 14 September 2010.
- ^ "Guernsey makes Mephedrone class B drug". BBC News. 13 April 2010. Retrieved 14 September 2010.
- ^ Campbell A (4 March 2010). "Call to ban legal high mephedrone test change". BBC Newsbeat. Retrieved 17 March 2010.
- ^ "Dopuna popisa opojnih droga, psihotropnih tvari i biljaka iz kojih se može dobiti opojna droga te tvari koje se mogu uporabiti za izradu opojnih droga" [List of narcotic drugs, psychotropic substances and plants from which narcotic drugs and substances that may be used to manufacture drugs can be obtained] (in Croatian). Croatian Ministry of Health and Social Welfare. 28 December 2009. Retrieved 4 July 2010.
- ^ "Vierundzwanzigste Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften" [24th Amendment of narcotics legislation] (in German). Selected laws in Germany – Buzer.de. 22 January 2010. Retrieved 4 July 2010.
- ^ Romanian Health Ministry (10 February 2010). "Comunicat de presă Ministerul Sănătăţii a stabilit lista cu plante şi substanţe cu proprietăţi psihoactive care vor fi interzise, după ce s-au dovedit a fi periculoase pentru sănătate" [Press release: The Health Ministry has established a list of plants and other substances with psychoactive properties that will be banned, after it has been proven that they are dangerous to health] (in Romanian). Romanian Health Ministry. Archived from the original on 10 February 2011. Retrieved 21 September 2010.
- ^ "Now illegal to import drug 'plant food' to Isle of Man". Isle of Man Today. 25 February 2010. Archived from the original on 28 February 2010. Retrieved 4 July 2010.
- ^ "Club drug mephedrone faces ban in U.K." CBC News. 29 March 2010. Retrieved 18 March 2014.
- ^ "8. Description of the control measures that are applicable to mephedrone in the Member States". Drugtext.org. Archived from the original on 10 December 2014. Retrieved 18 March 2014.
- ^ Ilston G (1 April 2010). "Mephedrone to be classified a Class B drug". Police Professional. Archived from the original on 15 July 2011. Retrieved 21 September 2010.
- ^ "BBC – Democracy Live – MPs move to ban mephedrone". BBC News. 7 April 2010. Retrieved 8 April 2010.
- ^ "The Misuse of Drugs (Amendment) (England, Wales and Scotland) Regulations 2010 No. 1144". Office of Public Sector Information. 16 April 2010. Archived from the original on 5 January 2011. Retrieved 8 April 2010.
- ^ Cumbow K (6 February 2011). "Synthetic form of cocaine and methamphetamine being packaged as bath salts". The Huntsville Times. Retrieved 10 February 2011.
- ^ Kelly F (3 March 2010). "Head shop substances to be banned". Irish Independent. Retrieved 21 September 2010.
- ^ "Minister for Health and Children announces immediate criminal ban on list of head shop products". Irish Department of Health and Children. 11 May 2010. Archived from the original on 21 February 2011. Retrieved 22 August 2010.
- ^ Gartland F (6 November 2009). "Irish youth are fourth highest cocaine users in Europe". The Irish Times. Archived from the original on 23 October 2010. Retrieved 21 September 2010.
- ^ "Arrêté royal du 13 Juin 2010 portant modification de l'arrêté royal du 22 janvier 1998 réglementant certaines substances psychotropes, et relatif à la réduction des risques et à l'avis thérapeutique" [Royal Decree of 13 June 2010 amending the Royal Decree of 22 January 1998 regulating certain psychotropic substances, therapeutic advice and on reducing risk] (in French). Belgian Department of Justice. 13 June 2010. Retrieved 5 July 2010.
- ^ "Tabelle sostanze stupefacenti e psicotrope" [Table of drugs and psychotropics] (in Italian). Italian Ministry of Health. 10 June 2010. Archived from the original on 27 June 2011. Retrieved 2 January 2011.
- ^ Ministry of Health of the Republic of Lithuania (18 June 2010). "2000 m. sausio 6 d. įsakymo nr. 5 "dėl narkotinių ir psichotropinių medžiagų sąrašų patvirtinimo" pakeitimo" [6 January 2000 Order no. 5 of narcotic drugs and psychotropic substances list] (in Lithuanian). Archived from the original on 30 November 2020. Retrieved 21 September 2010.
- ^ AFP (11 June 2010). "Classement comme stupéfiant de la méphédrone, dérivée du khat" [Classification of mephedrone from khat as a narcotic] (in French). france24.com. Archived from the original on 16 June 2010. Retrieved 4 July 2010.
- ^ "La méphédrone classée comme stupéfiant" [Mephedrone classified as a narcotic] (in French). French Ministry of Health and Sports. 11 June 2010. Archived from the original on 20 October 2010. Retrieved 26 October 2010.
- ^ "Ti nye stoffer på narkotikalisten" [Ten new compounds for drug list] (in Norwegian). Norwegian Broadcasting Corporation (NRK). 11 June 2010. Archived from the original on 14 June 2010. Retrieved 31 August 2010.
- ^ Government of the Russian Federation (29 July 2010). Постановление от 29 июля 2010 г. №578 [Decree of 29 July 2010 number 578] (in Russian). Archived from the original on 24 July 2011. Retrieved 19 November 2010.
- ^ "Mode-Droge Mephedron in Österreich verboten" [Fashionable drug mephedrone illegal in Austria] (in German). Die Presse. 22 August 2010. Archived from the original on 11 October 2012. Retrieved 22 August 2010.
- ^ "Ustawa z dnia 10 czerwca 2010 r. o zmianie ustawy o przeciwdziałaniu narkomanii" [The Law of 10 June 2010 amending the Act on Counteracting Drug Addiction] (in Polish). Polish Internet Database System of Legal Acts. 10 June 2010. Retrieved 25 August 2010.
- ^ Loh L (24 February 2010). "Legal in Singapore – the party drug that's banned everywhere else". Cnngo.com. Archived from the original on 29 June 2010. Retrieved 4 July 2010.
- ^ Yeo A (13 November 2010). "Three synthetic drugs to be banned". Todayonline. Archived from the original on 28 January 2011. Retrieved 16 November 2010.
- ^ Hermsmeier K (28 August 2010). "Ich verfiel der Partydroge "Meow"" [I fell into the party drug "Meow"]. Archived from the original on 19 July 2011. Retrieved 21 September 2010.
- ^ a b Impey J (3 December 2010). "EU bans 'meow meow' party drug". Deutsche Welle. Archived from the original on 9 February 2011. Retrieved 19 December 2010.
- ^ "Eldőlt: betiltják a katit" [Decided: cathinones banned] (in Hungarian). 2 September 2010. Retrieved 2 January 2011.
- ^ "Magyar közlöny 2010. december 15. Kormányrendelet a Mephedrone listáravételéről" [Hungarian Government bans Mephedrone] (PDF) (in Hungarian). 15 December 2010. Retrieved 24 January 2011.
- ^ "Documento BOE-A-2011-2490". BOE.es. pp. 13595–13596. Retrieved 18 March 2014.
- ^ "DECRETO por el que se reforman las fracciones I y III del artículo 245 de la Ley General de Salud". Diario Oficial de la Federación (in Spanish). Secretaría de Gobernación. 7 January 2014. Retrieved 1 September 2014.
- ^ "Mexico: mephedrone, TFMPP and synthetic cannabinoids placed under control". United Nations Office on Drugs and Crime. United Nations. January 2014. Retrieved 1 September 2014.
- ^ a b Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
- ^ "'Miaow' drug seized in mail busts". Sydney Morning Herald. 12 February 2010. Archived from the original on 1 August 2010. Retrieved 21 September 2010.
- ^ Levy M (22 January 2011). "Judge puts users of new drug on notice". The Age. Retrieved 17 February 2011.
- ^ "Misuse of Drugs Act 1975 No 116, Public Act – New Zealand Legislation". New Zealand Ministry of Health. 1 December 2009. Archived from the original on 19 May 2011. Retrieved 4 July 2010.
- ^ Edens J (14 September 2010). "Low-quality drugs prompt risk warning". The Southland Times. Archived from the original on 16 September 2010. Retrieved 21 September 2010.
- ^ "Controlled Drugs and Substances Act (1996, c. 19)". Canadian Department of Justice. 20 June 1996. Archived from the original on 6 January 2011. Retrieved 29 January 2011.
- ^ Chan K (18 March 2010). "Mephedrone: From plant food to Britain's party drug". Globe and Mail. Archived from the original on 29 June 2010. Retrieved 23 March 2010.
- ^ Elwell A (June 2010). "Britain moves to curtail new drug craze" (PDF). Primary. CMAJ. 182 (9): E393-4. doi:10.1503/cmaj.109-3245. PMC 2882479. PMID 20421358.
- ^ "U.K. party drug hits St. John's streets". CBC News. 25 June 2010. Retrieved 22 August 2010.
- ^ McCallum J (18 August 2010). "'Legal ecstasy' causing concern in Quebec". The Montreal Gazette. Archived from the original on 22 August 2010. Retrieved 22 August 2010.
- ^ "RNC seizes designer drug". The Telegram. 25 June 2010. Retrieved 22 August 2010.
- ^ "United States Drug Enforcement Administration Drug Scheduling". Justice.gov. Archived from the original on 7 February 2011. Retrieved 4 July 2010.
- ^ "Drugs and Chemicals of Concern – 4-Methylmethcathinone". United States Drug Enforcement Administration. July 2010. Archived from the original on 13 December 2010. Retrieved 21 September 2010.
- ^ a b Sewell A (28 January 2011). "'Bath salts' latest drug to raise alarms". Los Angeles Times. Archived from the original on 16 February 2011. Retrieved 17 February 2011.
- ^ "New York State Health Commissioner Bans Sale and Distribution of Dangerous Substances Marketed as Bath Salts". New York State Health Department. 23 May 2011. Retrieved 22 June 2011.
- ^ Ferran L. (7 September 2011). "DEA Announces Emergency Ban on 'Bath Salts'". ABC News. Retrieved 8 September 2011.
- ^ Keim B (12 July 2012). "New Federal Ban on Synthetic Drugs Already Obsolete". Wired. Retrieved 18 March 2014.
- ^ McNamara S, Stokes S, Coleman N (May 2010). "Head shop compound abuse amongst attendees of the Drug Treatment Centre Board". Primary. Irish Medical Journal. 103 (5): 134, 136–7. PMID 20666082. Archived from the original on 21 July 2011.
- ^ Saner E (5 December 2009). "Mephedrone and the problem with 'legal highs'". The Guardian. London. Retrieved 20 September 2010.
External links
[edit]- European Monitoring Center for Drugs and Drug Addiction (2011). Report on the risk assessment of mephedrone in the framework of the Council Decision on new psychoactive substances (Report). Publications Office. doi:10.2810/40800.
- Erowid 4-Methylmethcathinone Vault
- Mephedrone – Frequently asked questions www.lifeline.org.uk
- Guardian Daily Podcast: How dangerous is mephedrone?
- ChemSub Online: Mephedrone
