Higenamine
This article needs more reliable medical references for verification or relies too heavily on primary sources. (December 2018) |  |

| |
| Names | |
|---|---|
| IUPAC name
1-[(4-Hydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol
| |
| Other names
norcoclaurine, demethylcoclaurine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | higenamine |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H17NO3 | |
| Molar mass | 271.316 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including Nandina domestica (fruit), Aconitum carmichaelii (root), Asarum heterotropioides, Galium divaricatum (stem and vine), Annona squamosa, and Nelumbo nucifera (lotus seeds).
Higenamine is found as an ingredient in sports and weight loss dietary supplements sold in the US.[1] The US Food and Drug Administration has received reports of adverse effects from higenamine-containing supplements since 2014, but higenamine's health risks remain poorly understood.[1]
Legality
[edit]Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. Its main use is within food supplements developed for weight management and sports supplements.[1] Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies comparing the safety of modern formulations (based on synthetic higenamine) with traditional formulations. Nevertheless, it will not be added to the EU 'novel foods' catalogue, which details all food supplements that require a safety assessment certificate before use.[2]
Along with many other β2 agonists, higenamine is prohibited by World Anti-Doping Agency for use in sports.[3] In 2016, French footballer Mamadou Sakho was temporarily banned by UEFA after testing positive for Higenamine causing the player to miss the 2016 Europa League final. The ban was lifted after the player successfully made the mitigating defence that there was an absence of significant negligence as the substance was not on the list of banned substances despite drugs of the same category – β2 agonists – being banned.[4][5][6][7]
Pharmacology
[edit]Since higenamine is present in plants which have a history of use in traditional medicine, the pharmacology of this compound has attracted scientific interest.
In animal models, higenamine has been demonstrated to be a β2 adrenoreceptor agonist.[8][9][10][11][12] Adrenergic receptors, or adrenoceptors, belong to the class of G protein–coupled receptors, and are the most prominent receptors in the adipose membrane, besides also being expressed in skeletal muscle tissue. These adipose membrane receptors are classified as either α or β adrenoceptors. Although these adrenoceptors share the same messenger, cyclic adenosine monophosphate (cAMP), the specific transduction pathway depends on the receptor type (α or β). Higenamine partly exerts its actions by the activation of an enzyme, adenylate cyclase, responsible for boosting the cellular concentrations of the adrenergic second messenger, cAMP.[13]
In a rodent model, it was found that higenamine produced cardiotonic, vascular relaxation, and bronchodilator effects.[14][15] In particular, higenamine, via a beta-adrenoceptor mechanism, induced relaxation in rat corpus cavernosum, leading to improved vasodilation and erectile function.
Related to improved vasodilatory signals, higenamine has been shown in animal models to possess antiplatelet and antithrombotic activity via a cAMP-dependent pathway, suggesting higenamine may contribute to enhanced vasodilation and arterial integrity.[8][13][15][16]
In humans, higenamine has been studied as an investigational drug in China for use as a pharmacological agent for cardiac stress tests as well as for treatment of a number of cardiac conditions including bradyarrhythmias.[1] The human trials were relatively small (ranging from 10 to 120 subjects) and higenamine was administered intravenously, most commonly using gradual infusions of 2.5 or 5 mg.[1] Higenamine consistently increased heart rate but had variable effects on blood pressure. One small study described higenamine's effect on cardiac output: higenamine led to an increased ejection fraction in 15 patients with heart disease.[1]
Toxicity
[edit]The safety of orally administered higenamine in humans is unknown. During a study of acute toxicity, mice were orally administered the compound at a dose of 2 g per kg of bodyweight. No mice died during the study.[17] In human trials of intravenous higenamine, subjects who received higenamine reported shortness of breath, racing heart, dizziness, headaches, chest tightness.[1]
Biosynthesis
[edit](S)-Norcoclaurine/Higenamine is at the center of benzylisoquinoline alkaloid (BIA) biosynthesis. In spite of large structure diversity, BIAs biosynthesis all share a common first committed intermediate (S)-norcoclaurine.[18] (S)-norcoclaurine is produced by the condensation of two tyrosine derivatives, dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA).
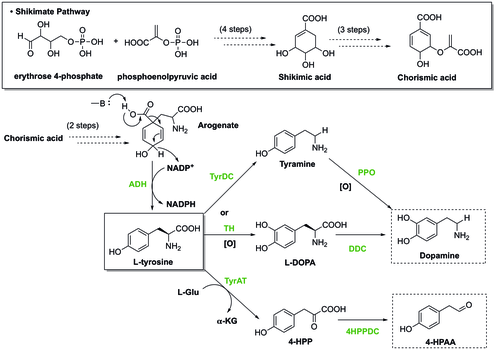
In plants, tyrosine is synthesized through Shikimate pathway, during which the last step involves decarboxylation and dehydrogenation of arogenate to give L-tyrosine. To generate dopamine from tyrosine, there are two pathways. In one pathway, tyrosine undergoes decarboxylation catalyzed by tyrosine decarboxylase (TyrDC) to become tyramine, which is then followed by oxidation of polyphenol oxidase (PPO) to render dopamine.[19][20] Alternatively, tyrosine can be oxidized by tyrosine hydroxylase (TH) to form L-DOPA, which is then later decarboxylated by DOPA decarboxylase (DDC) to provide dopamine. Besides that, the other starting material, 4-HPAA, is generated through a first transamination by tyrosine transeaminase (TyrAT) to form 4-hydroxylphenylpyruvate (4-HPP), and a subsequent decarboxylation by 4-HPP decarboxylase.[20]

The condensation of dopamine and 4-HPAA to form (S)-norcoclaurine is catalyzed by (S)-norcoclaurine synthase (NCS).[21] Such reaction is one type of Pictet-Spengler reaction. In this reaction, Asp-141 and Glu-110 in the NCS active site are involved in the activation of the amine and carbonyl respectively to facilitate imine formation. Then, the molecule will be cyclized as the mechanism shown below to produce (S)-nococlaurine.
See also
[edit]References
[edit]- ^ a b c d e f g Cohen, Pieter A.; Travis, John C.; Keizers, Peter H. J.; Boyer, Frederick E.; Venhuis, Bastiaan J. (6 September 2018). "The stimulant higenamine in weight loss and sports supplements". Clinical Toxicology. 57 (2): 125–130. doi:10.1080/15563650.2018.1497171. PMID 30188222. S2CID 52165506.
- ^ "Novel food catalogue". Food Safety. European Commission.
- ^ "Prohibited Substances at All Times". List of Prohibited Substances and Methods. World Anti-Doping Agency. 1 January 2016. Archived from the original on 20 August 2016. Retrieved 21 August 2016.
- ^ "Mamadou Sakho: Liverpool defender investigated over failed drugs test". BBC. 23 April 2016.
- ^ "Euro 2016: Mamadou Sakho could play for France as Uefa opts not to extend ban". BBC. 28 May 2016.
- ^ "Mamadou Sakho - UEFA decision raises key questions". Echo. 28 May 2016.
- ^ "Mamadou Sakho still set to miss EURO 2016, despite being cleared of doping". Get French Football. 29 May 2016.
- ^ a b Tsukiyama M, Ueki T, Yasuda Y, Kikuchi H, Akaishi T, Okumura H, Abe K (October 2009). "Beta2-adrenoceptor-mediated tracheal relaxation induced by higenamine from Nandina domestica Thunberg". Planta Medica. 75 (13): 1393–9. doi:10.1055/s-0029-1185743. PMID 19468973. S2CID 260280804.
- ^ Kashiwada Y, Aoshima A, Ikeshiro Y, Chen YP, Furukawa H, Itoigawa M, Fujioka T, Mihashi K, Cosentino LM, Morris-Natschke SL, Lee KH (January 2005). "Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids". Bioorganic & Medicinal Chemistry. 13 (2): 443–8. doi:10.1016/j.bmc.2004.10.020. PMID 15598565.
- ^ Kimura I, Chui LH, Fujitani K, Kikuchi T, Kimura M (May 1989). "Inotropic effects of (+/-)-higenamine and its chemically related components, (+)-R-coclaurine and (+)-S-reticuline, contained in the traditional sino-Japanese medicines "bushi" and "shin-i" in isolated guinea pig papillary muscle". Japanese Journal of Pharmacology. 50 (1): 75–8. doi:10.1254/jjp.50.75. PMID 2724702.
- ^ Kang YJ, Lee YS, Lee GW, Lee DH, Ryu JC, Yun-Choi HS, Chang KC (October 1999). "Inhibition of activation of nuclear factor kappaB is responsible for inhibition of inducible nitric oxide synthase expression by higenamine, an active component of aconite root". The Journal of Pharmacology and Experimental Therapeutics. 291 (1): 314–20. PMID 10490919.
- ^ Yun-Choi HS, Pyo MK, Park KM, Chang KC, Lee DH (October 2001). "Anti-thrombotic effects of higenamine". Planta Medica. 67 (7): 619–22. doi:10.1055/s-2001-17361. PMID 11582538. S2CID 260279615.
- ^ a b Kam SC, Do JM, Choi JH, Jeon BT, Roh GS, Chang KC, Hyun JS (2012). "The relaxation effect and mechanism of action of higenamine in the rat corpus cavernosum". International Journal of Impotence Research. 24 (2): 77–83. doi:10.1038/ijir.2011.48. PMID 21956762.
- ^ Bai G, Yang Y, Shi Q, Liu Z, Zhang Q, Zhu YY (October 2008). "Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist1". Acta Pharmacologica Sinica. 29 (10): 1187–94. doi:10.1111/j.1745-7254.2008.00859.x. PMID 18817623.
- ^ a b Pyo MK, Lee DH, Kim DH, Lee JH, Moon JC, Chang KC, Yun-Choi HS (July 2008). "Enantioselective synthesis of (R)-(+)- and (S)-(-)-higenamine and their analogues with effects on platelet aggregation and experimental animal model of disseminated intravascular coagulation". Bioorganic & Medicinal Chemistry Letters. 18 (14): 4110–4. doi:10.1016/j.bmcl.2008.05.094. PMID 18556200.
- ^ Liu W, Sato Y, Hosoda Y, Hirasawa K, Hanai H (November 2000). "Effects of higenamine on regulation of ion transport in guinea pig distal colon". Japanese Journal of Pharmacology. 84 (3): 244–51. doi:10.1254/jjp.84.244. PMID 11138724.
- ^ Lo CF, Chen CM (February 1997). "Acute toxicity of higenamine in mice". Planta Medica. 63 (1): 95–6. doi:10.1055/s-2006-957619. PMID 9063102. S2CID 260281301.
- ^ Hagel JM, Facchini PJ (May 2013). "Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new world". Plant & Cell Physiology. 54 (5): 647–72. doi:10.1093/pcp/pct020. PMID 23385146.
- ^ Soares AR, Marchiosi R, Siqueira-Soares RC, Barbosa de Lima R, Marchiosi R, Dantas dos Santos W, Ferrarese-Filho O (March 2014). "The role of L-DOPA in plants". Plant Signaling & Behavior. 9 (4): e28275. Bibcode:2014PlSiB...9E8275S. doi:10.4161/psb.28275. PMC 4091518. PMID 24598311.
- ^ a b Beaudoin GA, Facchini PJ (July 2014). "Benzylisoquinoline alkaloid biosynthesis in opium poppy". Planta. 240 (1): 19–32. Bibcode:2014Plant.240...19B. doi:10.1007/s00425-014-2056-8. PMID 24671624.
- ^ Lichman BR, Sula A, Pesnot T, Hailes HC, Ward JM, Keep NH (October 2017). "Structural Evidence for the Dopamine-First Mechanism of Norcoclaurine Synthase". Biochemistry. 56 (40): 5274–5277. doi:10.1021/acs.biochem.7b00769. PMC 5637010. PMID 28915025.
