Wikipedia talk:WikiProject Elements/Archive 39
| This is an archive of past discussions about Wikipedia:WikiProject Elements. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 35 | ← | Archive 37 | Archive 38 | Archive 39 | Archive 40 | Archive 41 | → | Archive 45 |
The Redirects
I've noticed on the article lists I see Helium-2 and Helium-3, but I also saw Deuterium and tritium. They're both the same they just redirect to each other. We should make it so it recognizes it.--Julianstout (talk) 22:51, 7 March 2019 (UTC)
- @Julianstout: Did you mean hydrogen-2 and hydrogen-3? And what exactly are you asking about the redirects? ComplexRational (talk) 01:05, 8 March 2019 (UTC)
- Deuterium and tritium are isotopes of hydrogen, not helium. So helium-2 and helium-3 are, and should remain, separate articles with no redirects. The articles for hydrogen-2 and hydrogen-3 redirect to deuterium and tritium in case anyone is searching for them under that title. ― Дрейгорич / Dreigorich Talk 12:56, 8 March 2019 (UTC)
- These are the pages (you won't be redirected):
- Hydrogen-1
- Hydrogen-2
- Hydrogen-3
- Deuterium
- Tritium
- Hydrogen
- Category:Isotopes of hydrogen (16)
- Protium (because of this section title: Isotopes of hydrogen#Hydrogen-1 (protium)
- Protium (isotope) -- created; redirect to hydrogen-1. This way the name appears in the category.
- Protonium
- -DePiep (talk) 13:28, 8 March 2019 (UTC)
- Actually, protium is hydrogen-1. Protonium is something different (an exotic atom consisting of a proton and an antiproton). Double sharp (talk) 13:44, 8 March 2019 (UTC)
- OK, Adjusted... - DePiep (talk) 13:50, 8 March 2019 (UTC)
- Actually, protium is hydrogen-1. Protonium is something different (an exotic atom consisting of a proton and an antiproton). Double sharp (talk) 13:44, 8 March 2019 (UTC)
Project MOS/Guidelines
I think it would be good to have a WP:ELEM/MOS or WP:ELEM/Guidelines page to collect our project-specific style guidelines. Over the years, we've had lots of discussions and reached many collaborative decisions, but to my knowledge, we have no place to gather these decisions into an easily referenced list. The first step would be to gather up such a list. This would include, for example,
- Names of elements (hydrogen, helium) are common nouns and never capitalized just because they are elements.
- Element symbols (H, He) always begin with an upper case letter
This is the sort of thing I'm talking about, but these were not decided by our project. Can anyone think of anything we've decided on that should be documented? YBG (talk) 05:04, 21 February 2019 (UTC)
- Well, we do have Wikipedia:WikiProject Elements/Guidelines, but that is positively ancient. I think your ideas posted at its talk page would be very good, although I can't think of very many things that really need to be codified that haven't been codified elsewhere (among those you mention, spelling is at WP:ALUM, infoboxes for elements are basically common sense, so that just leaves a specific statement of how notability ought to apply to undiscovered elements). Double sharp (talk) 06:57, 21 February 2019 (UTC)
- That's the 2nd time today I've encountered talk page comments that I wrote but now have no recollection about. As I don't have an ancient keyboard to blame it on, it may be an indication that it is time for my annual wikibreak. YBG (talk) 08:10, 21 February 2019 (UTC)
- (For context, see my edit summary to the above comment: sorry if I typo more than usual; I just replaced my ancient keyboard ^_^.)
- It's fine, we all forget things sometimes. The good thing about writing them up as comments here is that they are still here even you've forgotten that you'd thought of them. But if you do want to take a wikibreak, I hope you come back very refreshed! ^_^ Double sharp (talk) 10:14, 21 February 2019 (UTC)
- @Double sharp: I support the inclusion of a standard for the notability of undiscovered elements. There have been many debates on the inclusion of such articles, especially E123, always reaching the same conclusion. If we lay out the notability standard now, there would be no need to discuss the subject on this talk page (we can direct anyone who asks to this proposed guideline), unless new research is published/discovered that might fulfill WP:GNG and/or a new consensus forms.
- Also pinging @YBG: ComplexRational (talk) 15:24, 21 February 2019 (UTC)
- Also, do we have a notability guideline aside from GNG for individual isotopes? I don't know of any standard, and given the quantity of available sources on all 3000 or so isotopes, it's not clear when an isotope page should redirect/redirect to a section in [[isotopes of element]] or have a standalone article. ComplexRational (talk) 15:20, 21 February 2019 (UTC)
- I think, though, that isotopes would be treated in a similar manner to astronomical objects - as both are relatively obscure but covered in many sources (discovery papers, catalogs). Some isotopes without articles also have practical applications or a special relevance in physics that are documented in various sources.
- One other idea: There doesn't appear to be consistent usage of various templates such as {{SimpleNuclide2}}, {{Isotopes table/footer}} (but not {{Isotopes table/header}}), {{val}} (especially in isotope pages) – would a set of guidelines outlining how and when to use certain templates be a good idea? ComplexRational (talk) 15:49, 21 February 2019 (UTC)
- TL;DR: build strong pages both WP:MOS/Chemistry and WP:ELEM/Guidelines. By collecting existing styles we use, and discuss new ones.
- In my view, a MOS is much stronger, more lawful and more generic (Wiki-wide) than WP:ELEMENTS/guidelines. We could expand WP:MOS/Chemistry with more element issues like WP:ALUM -- which is already in a MOS btw; on how we source properties like m.p. and b.p. [data page]), notability of elements, isotopes. We could also create our "own" page WP:MOS/chemical elements, which would be just as good. Next to that, we can update and maintain Wikipedia:WikiProject Elements/Guidelines for more local issues (preferred article structure, consistent use of periodic table data & presentation, document our category classification system, help-like sections). Both pages best be a repository for styles we use in all WP:ELEM (and were most often discussed througly, into a stable result).
- As for notability of isotopes: any isotope with substantial material (sourced texts) can be an article IMO. I don't know if a general guideline for such creation is feasible (too much situations?). Easy way to go: an article can nicely grow from a dedicated section in the Isotopes of E article, building its merit & quality in there.
- I support updating both MOS:CHEM with most relevant/perennial topics and WP:ELEM/Guidelines for everything else. ComplexRational (talk) 22:02, 22 February 2019 (UTC)
- After rereading the MOS page, it looks like such information would fit perfectly as a subsection entitled Elements of MOS:CHEM#Article types, which can also include a note on hypothetical element articles. Maybe merge the existing content (or a reformatted version of it) from WP:ELEM/Guidelines? ComplexRational (talk) 22:20, 22 February 2019 (UTC)
- @DePiep and YBG: I have boldly attempted to summarize the consensus for notability of undiscovered elements at Wikipedia:Notability (chemicals). ComplexRational (talk) 19:02, 9 March 2019 (UTC)
- That's the 2nd time today I've encountered talk page comments that I wrote but now have no recollection about. As I don't have an ancient keyboard to blame it on, it may be an indication that it is time for my annual wikibreak. YBG (talk) 08:10, 21 February 2019 (UTC)
Side note on isotopes table templates
- (Detail: I started {{Isotopes table/header}} some time ago to standardise the Big Isotopes Table header + ease of maintenance. However, it appeared that the 118+ headers varied too much between them, so I postponed that enterprise, until I found more urgency in my agenda. The /footer was left behind as an orphan). -DePiep (talk) 10:13, 22 February 2019 (UTC)
- @DePiep: As I understand it, there are only three major differences. These are: natural (H-Mo, Ru-Nd, Sm-U) and artificial (Tc, Pm, Np+) element, which concerns the two rightmost columns (natural abundance); historic name, for one of the columns in the tables for Hg-U; existence of nuclear isomers and the display of the 3-wide excitation energy column. I don't know the exact syntax, but is it possible to configure boolean parameters for natural/artificial, historic names/not, and isomers/not for the different headings? If this is too difficult to handle now, I'm fine leaving it as is and doing periodic maintenance manually. ComplexRational (talk) 22:02, 22 February 2019 (UTC)
- (Detail: I started {{Isotopes table/header}} some time ago to standardise the Big Isotopes Table header + ease of maintenance. However, it appeared that the 118+ headers varied too much between them, so I postponed that enterprise, until I found more urgency in my agenda. The /footer was left behind as an orphan). -DePiep (talk) 10:13, 22 February 2019 (UTC)
- I will take a look at this. Technically, I'll first make these headers into a few templates—as they are. Once in template, we can more easily improve the headers & footnotes (maintenance). An other task would be: make each row (isotope entrance) a template, like we have in infoboxes, e.g. {{infobox element isotopes/isotopes decay3}}. However, this can only be done later. If you are fine working with today's table pipe symbols "|" etc., as you say, that is the most stable way. -DePiep (talk) 10:46, 2 March 2019 (UTC)
- @DePiep: Sounds good. And I can handle pipe symbols for now. ComplexRational (talk) 18:11, 2 March 2019 (UTC)
- Developments now continue at {{Isotopes table}}. Mostly table-technical issues for now. Content improvements in all lists of Category:Lists of isotopes by element (122), including sourcing, are supported and greatly appreciated (ComplexRational is doing a great job in this). Goal: make the enwiki isotope data best, and present them in a very nice table :-). -DePiep (talk) 15:29, 3 March 2019 (UTC)
- @DePiep: Sounds good. And I can handle pipe symbols for now. ComplexRational (talk) 18:11, 2 March 2019 (UTC)
Inconsistent half-lives of superheavy elements
Following a discussion with RockMagnetist at Talk:Island of Stability#Half-life table, it came to light that half-lives for isotopes of superheavy elements from {{NUBASE 2016}} and various journal articles (e.g. [t 1][t 2][t 3]) are quite inconsistent. This became evident when I realized that, according to the most recent papers and opposite to what our articles say, 269Hs is more stable than 270Hs. While there is generally agreement on which isotopes are the most stable (including 269Hs), some half-lives differ by a factor of 4 even when there is no new data since 2016. In island of stability, we decided to list both sets of values to demonstrate variation in the data (and comply with WP:NPOV), though I would hesitate to list multiple values in isotope tables or infoboxes or have dozens of footnotes explaining different values, as that would probably confuse many readers.
@Double sharp, YBG, R8R, DePiep, and Sandbh: Which data should be listed in such tables, especially for hassium and isotopes of hassium (which are the priority, but with which numbers)? I personally prefer recent journal articles (especially those published later than {{NUBASE 2016}})—in which case, less fixing is needed—though all other isotope articles mostly use NUBASE as a standard. ComplexRational (talk) 17:05, 9 March 2019 (UTC)
- A clear description of the issue, and actually just a fact of evolving science, ie new data, we must be able to take care of. That's our job at the encyclopedia. (Hell, even the standard atomic weights still change). So we are talking about values varying over RS's. It is up to editors like you to declare these values OK, as is common WP practice.
- The Big Table of Isotopes (BToI): I think all these varying values and their background should be presented in the Big Table of Isotopes (like Isotopes of hassium#List of isotopes). In there, no reader will complain about data or sourcing being "too complex". There one can add all sources, descriptive footnotes (like {{efn}}), we can add a column "Notes" to the table for a short description. Formatted, writing two half-lifes could look like this for 269Hs:
| 16 s[1]<br/>''or''<br/>9.7 s[2]{{efn|The sources give different values. There is ...[longer footnote here]}}.- You can also add a dedicated section to that article. Maybe after some time, the situation will be solved and we can adjust the table (the issue itself has a half-life ;-) ).
- In short: in the BToI you are unrestricted in adding this information (limits are elsewhere, like quality).
- In {{Infobox hassium isotopes}}: two values would be too much detail IMO. Choose one, defendable but without defending (explaining) it in there. Then #link to the BToI on that page! (use an anchor like <sup>[[#Hs-269|details]]</sup>?). One link is maximum in these infoboxes.
- In {{Infobox hassium}}, Main isotopes: first of all, any important isotope will be handled in the body text first. There is enough space for clarifying text, and it can have a #-link to the Big Table nicely. In the Infobox only one value, maybe without any extra link at all (bodytext should do, In general, the Infobox should not introduce new information by itself).
References
- ^ Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- ^ Oganessian et al., 2015
New table for isotopes
- @DePiep: I created two new templates, {{Isotopes summary}} and {{Isotopes summary/isotope}}. These include two columns in the same format as the list in island of stability and several common references; this can create a new standard for the isotope tables in the main element articles. ComplexRational (talk) 15:06, 10 March 2019 (UTC)
- Nice! Am I supposed to do anything? You expect wider usage? -DePiep (talk) 15:09, 10 March 2019 (UTC)
- As of right now, the articles for elements 104 to 112 use such a table, and there are similar lists in a handful of other pages. It could also be used in the articles for 113 to 118, and maybe 101 to 103. Does AWB allow you to quickly replace the existing tables with these templates, or need it be done manually? In either case, I need to check all the values first and add/update anything that is missing. ComplexRational (talk) 15:16, 10 March 2019 (UTC)
- Nice! Am I supposed to do anything? You expect wider usage? -DePiep (talk) 15:09, 10 March 2019 (UTC)
- @DePiep: I created two new templates, {{Isotopes summary}} and {{Isotopes summary/isotope}}. These include two columns in the same format as the list in island of stability and several common references; this can create a new standard for the isotope tables in the main element articles. ComplexRational (talk) 15:06, 10 March 2019 (UTC)
- You want to replace the Big Table of Isotopes with these (like in Sg, Hs)? Or should it be added to the page? -DePiep (talk) 15:27, 10 March 2019 (UTC)
- I am not very familiar with isotopes. I do not automatically see where this new table belongs ;-) -DePiep (talk) 15:50, 10 March 2019 (UTC)
- @DePiep: See the small table in Copernicium#Isotopes as an example (just updated with these templates). That's where they should go. ComplexRational (talk) 15:58, 10 March 2019 (UTC)
- I see, in the articles! Little to do for AWB, because it is not a replacement. Adding a blank templates for you to fill will look bad at first. For now, I cannot help in this I guess. Have a nice edit. -DePiep (talk) 16:21, 10 March 2019 (UTC)
Oxidation state
From Template talk: ... oxidation-state, here for wider audience:
I thought that the group II oxides were weak bases, but obviously that depends on what you compare them with. Magnesium oxide (hydroxide in solution) is milk of magnesia, commonly used as an oral medicine. I don't know about the ones farther down, but I would expect even less basic.
(ping Gah4) -DePiep (talk) 21:25, 10 March 2019 (UTC)
- Mg(OH)2 is only a weak base in solution because it is poorly soluble in water. Ca(OH)2, Sr(OH)2, and Ba(OH)2 are strong bases; only Be(OH)2 is amphoteric. Double sharp (talk) 13:49, 11 March 2019 (UTC)
"element with symbol" or "element with the symbol"
I had noticed that User:DePiep had changed the upcoming TFA on germanium to use the first wording and found that an odd phrasing. DePiep mentioned that this was the way all the element articles phrased it and said that I should bring the question here if I felt that that should change. I am now doing so.--Khajidha (talk) 16:10, 27 February 2019 (UTC)
- I personally think the element articles should read "with the symbol" instead, as it feels more natural to me too. On the other hand, it is a small difference and my opinion on it is not strong, because omitting the article does not sound really wrong to me either. Double sharp (talk) 03:44, 28 February 2019 (UTC)
- I also don't have a strong preference, but I lean slightly to omitting the article, mostly because there is no article in from of "atomic number" (yea, I know, that's just a hobgoblin). But regardless, I think it best to consider this issue by looking at the entire sentence:
- Germanium is a chemical element with symbol Ge and atomic number 32.
- Germanium is a chemical element with the symbol Ge and atomic number 32.
- YBG (talk) 04:57, 28 February 2019 (UTC)
- Here's an alternative:
- Germanium (symbol: Ge) is a chemical element with atomic number 32.
- YBG (talk) 06:01, 28 February 2019 (UTC)
- I like your alternative. --Khajidha (talk) 17:32, 28 February 2019 (UTC)
- @DePiep, Khajidha, Double sharp, Sandbh, Dank, David Levy, and ComplexRational: If we're going to change it to my alternative, now is the time to do it ... the FA goes live in a few hours. YBG (talk) 21:35, 28 February 2019 (UTC)
- Thoughts? - Dank (push to talk) 22:03, 28 February 2019 (UTC)
- @DePiep, Khajidha, Double sharp, Sandbh, Dank, David Levy, and ComplexRational: If we're going to change it to my alternative, now is the time to do it ... the FA goes live in a few hours. YBG (talk) 21:35, 28 February 2019 (UTC)
- I like your alternative. --Khajidha (talk) 17:32, 28 February 2019 (UTC)
- I also don't have a strong preference, but I lean slightly to omitting the article, mostly because there is no article in from of "atomic number" (yea, I know, that's just a hobgoblin). But regardless, I think it best to consider this issue by looking at the entire sentence:
- No. "The symbol" pertains to an existing symbol. But an element symbol is 'new'. -DePiep (talk) 22:44, 28 February 2019 (UTC)
- Also, YBG, this is not about a change in the TFA. It is about a change in all 118+ element articles. (TFA follows article). -DePiep (talk) 22:47, 28 February 2019 (UTC)
- Actually, I thought this discussion had to do with making the FA blurb acceptable to those who objected to the absence of "the". I was not offering my alternative as a suggestion to modify all 118+ articles, though that could be taken up separately.
- You say
"The symbol" pertains to an existing symbol. But an element symbol is 'new'.
In Article (grammar) § Definite article it saysThe definite article is used to refer to a particular member of a group or class. It may be something that the speaker has already mentioned or it may be something uniquely specified. There is one definite article in English, for both singular and plural nouns: the
.
- This explanation may be helpful to some people. YBG (talk) 23:22, 28 February 2019 (UTC)
- Also, YBG, this is not about a change in the TFA. It is about a change in all 118+ element articles. (TFA follows article). -DePiep (talk) 22:47, 28 February 2019 (UTC)
- Do not change the TFA. To change all 118+ articles, start a talk. -DePiep (talk) 23:28, 28 February 2019 (UTC)
- Since the element symbols are unique to each element, it seems clear from YBG's provided explanation that all 118+ articles should be changed to add "the". And it should be done as soon as possible, at least for Ge as first priority, because this is a matter of correct English. Double sharp (talk) 23:37, 28 February 2019 (UTC)
- No, again, to this: a symbol like 'Ge' is not an universal symbol, so not a 'the' symbol.
- THE symbol "
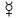 " was pre-known, universal, and re-used for both Mercury (planet) and Mercury (element) and more. -DePiep (talk) 23:53, 28 February 2019 (UTC)
" was pre-known, universal, and re-used for both Mercury (planet) and Mercury (element) and more. -DePiep (talk) 23:53, 28 February 2019 (UTC)
- Where are you getting this "universal symbol" requirement from? I've never heard of such a thing. And it seems neither has anyone else.--Khajidha (talk) 00:00, 1 March 2019 (UTC)
- "THE symbol x" is universal aka preknown aka existing aka generic aka ... . "Ge" is not, it was invented for Ge as a symbol and for Ge only. -DePiep (talk) 00:14, 1 March 2019 (UTC)
- Repeating it doesn't make it true. "The symbol $ represents the dollar". It's just basic English grammar. --Khajidha (talk) 00:17, 1 March 2019 (UTC)
- Duh? You asked for clarification, I clarified, then you say "repetition"???
- Anyway, "$" is a "THE symbol $" indeed (you self-contradict). Symbols are not grammar btw. "Ge" is *not* a THE symbol, it is new & unique and probably you did not even know it is a symbol. -DePiep (talk) 00:22, 1 March 2019 (UTC)
- You did not explain, you simply repeated your assertion. Where is this so called rule artuculated? I know quite well that it is a symbol, I lesrned that many yesrs ago in middle school. And the grammar comment was about the use of "the".
- I say: "THE symbol x" is an universal, existing symbol "x", and "symbol Ge" is incidental, unique, not existing and so not a "THE symbol". hth -DePiep (talk) 00:59, 1 March 2019 (UTC)
- Yes, YOU say. You have yet to answer my question of where you have encountered this rule or what language authority supports it. It is cintradicted by my entire experience with the discussion of symbols in English. --Khajidha (talk) 01:36, 1 March 2019 (UTC)
- Yes: I say. That is: I do not decide as a god, I try sincerely to explain what I mant to write. -DePiep (talk) 02:11, 1 March 2019 (UTC)
- Yes, YOU say. You have yet to answer my question of where you have encountered this rule or what language authority supports it. It is cintradicted by my entire experience with the discussion of symbols in English. --Khajidha (talk) 01:36, 1 March 2019 (UTC)
- I say: "THE symbol x" is an universal, existing symbol "x", and "symbol Ge" is incidental, unique, not existing and so not a "THE symbol". hth -DePiep (talk) 00:59, 1 March 2019 (UTC)
- You did not explain, you simply repeated your assertion. Where is this so called rule artuculated? I know quite well that it is a symbol, I lesrned that many yesrs ago in middle school. And the grammar comment was about the use of "the".
- Repeating it doesn't make it true. "The symbol $ represents the dollar". It's just basic English grammar. --Khajidha (talk) 00:17, 1 March 2019 (UTC)
- "THE symbol x" is universal aka preknown aka existing aka generic aka ... . "Ge" is not, it was invented for Ge as a symbol and for Ge only. -DePiep (talk) 00:14, 1 March 2019 (UTC)
- Where are you getting this "universal symbol" requirement from? I've never heard of such a thing. And it seems neither has anyone else.--Khajidha (talk) 00:00, 1 March 2019 (UTC)
- Since the element symbols are unique to each element, it seems clear from YBG's provided explanation that all 118+ articles should be changed to add "the". And it should be done as soon as possible, at least for Ge as first priority, because this is a matter of correct English. Double sharp (talk) 23:37, 28 February 2019 (UTC)
The edit that removed "the" was made on the 26th ... that's not enough time to know where the consensus lies (for the blurb, not the article). The second sentence of the blurb says "chemically similar to silicon (Si) and tin (Sn)". Since that's the format we're using in the blurb for silicon and tin, it makes sense to use that format for germanium too, and I just made the change. I'm not taking a position on "the". - Dank (push to talk) 00:28, 1 March 2019 (UTC)
- TFA blurb follows article. Article has no "THE", as no element articles has, good & consistent. Case closed. (If someone wants to change that: go ahead, propose it, for all 118+ element articles then). This is SOP for TFA, what else can I say. (Also, *if* this question were asked properly, you know my argument). -DePiep (talk) 00:46, 1 March 2019 (UTC)
- All right, I shall propose it then: Double sharp (talk) 02:03, 1 March 2019 (UTC)
- Well, depending on what timezone everybody is in, the FA is up in some parts of the world. Now, time to weigh in. Sure DePiep, Germanium may not have a symbol that is represented by a specific ASCII character as the ampersand and dollar sign do, but that does not take away from the fact that Germanium has a symbol, and that symbol is Ge. Even if it isn't one character, the two letters "GE" serve as the universal symbol for Ge. If this element were to be, say, Gormanium (which wouldn't happen because Gormany isn't a country), then the symbol for Gormanium would be Go instead of Ge. There is not any possible incident in such case that the symbol for Gormanium would be anything besides Go; much is the case where there lacks to be an incident in which Germanium's symbol is anything besides Ge. Just because a new character was created to be a symbol for an idea, (such that § was made to represent a section of writing,) doesn't imply that two existing letters can be made to form a symbol as well. It can just as well be a universal representative for the element Germanium. And yes, there can be a symbol that can represent multiple different things. GE is a symbol for General Electric as much as it is for Germanium; it all comes down to the context at that point.UtopianPoyzin (talk) 04:20, 1 March 2019 (UTC)
- Opening line from article The: "The is a grammatical article in English, denoting person(s) or thing(s) already mentioned, under discussion, implied, or otherwise presumed familiar to listeners or readers." That nicely describes it. (also re Khajidha). -DePiep (talk) 14:19, 1 March 2019 (UTC)
- And I'm not seeing how that supports your position. The sentence is discussing the specific symbol used for Germanium, that falls under the "under discussion" portion of the definition. --Khajidha (talk) 14:36, 1 March 2019 (UTC)
- PS-for the same reason, your post should read "The opening line from the article..." --Khajidha (talk) 14:38, 1 March 2019 (UTC)
- Opening line from article The: "The is a grammatical article in English, denoting person(s) or thing(s) already mentioned, under discussion, implied, or otherwise presumed familiar to listeners or readers." That nicely describes it. (also re Khajidha). -DePiep (talk) 14:19, 1 March 2019 (UTC)
- Well, depending on what timezone everybody is in, the FA is up in some parts of the world. Now, time to weigh in. Sure DePiep, Germanium may not have a symbol that is represented by a specific ASCII character as the ampersand and dollar sign do, but that does not take away from the fact that Germanium has a symbol, and that symbol is Ge. Even if it isn't one character, the two letters "GE" serve as the universal symbol for Ge. If this element were to be, say, Gormanium (which wouldn't happen because Gormany isn't a country), then the symbol for Gormanium would be Go instead of Ge. There is not any possible incident in such case that the symbol for Gormanium would be anything besides Go; much is the case where there lacks to be an incident in which Germanium's symbol is anything besides Ge. Just because a new character was created to be a symbol for an idea, (such that § was made to represent a section of writing,) doesn't imply that two existing letters can be made to form a symbol as well. It can just as well be a universal representative for the element Germanium. And yes, there can be a symbol that can represent multiple different things. GE is a symbol for General Electric as much as it is for Germanium; it all comes down to the context at that point.UtopianPoyzin (talk) 04:20, 1 March 2019 (UTC)
- All right, I shall propose it then: Double sharp (talk) 02:03, 1 March 2019 (UTC)
- re "how that supports your position": it is a plain argument against your OP. The article The, and Merriam Webster, clearly state that one can use the definite article "The" when the noun is already known. Of course, in the opening sentence we are introducing the symbol, which is the opposite of "known". HTH. -DePiep (talk) 11:42, 2 March 2019 (UTC)
Proposal: change "symbol X" to "the symbol X" in all element article lede sections
- Support per YBG's quote describing article usage in English; the article should be used as the symbol is unique to the element. Double sharp (talk) 02:03, 1 March 2019 (UTC)
- Oppose for all elements, per argumentation above. -DePiep (talk) 02:07, 1 March 2019 (UTC)
- It is not about whether "Ge" is a symbol of sorts. It is about using the definite article "The" here. From the article: '... denoting thing(s) already mentioned' while that is exactly not the case in the opening sentence: it is introducing the terms. See also Merriam Webster Online Dictionary.: [The] — used as a function word to indicate that a following noun or noun equivalent is definite or has been previously specified by context or by circumstance - DePiep (talk) 10:57, 2 March 2019 (UTC)
- And in this case, the symbol Ge is definite, even if it has not been previously been specified, so the article should be there. Our article on The also mentions in its first sentence: "The ... is a grammatical article in English, denoting persons or things already mentioned, under discussion [my emphasis], implied or otherwise presumed familiar to listeners or readers", and since the symbol (again) is under discussion, the article should again be there. Double sharp (talk) 13:47, 8 March 2019 (UTC)
- "the symbol Ge is definite" ? Can't find that in the source (Mirriam Webster), so its hard to grasp. Do you mean that symbol "Ge" is defined and unchanging in RL? In that case, the word 'definite' is not grammatical. 'Definite' in grammar (noun phrase) means: identified, not generic (my wording, see [2]). re "[symbol Ge is] under discussion": too much of a stretch. "Under discussion" looses to "being defined" in the article opening sentence. Further below indeed the naming & symbol can be "under discussion" in the article body ('the symbol Xx was proposed because ...'). The opening sentence should use MOS:THETITLE, requiring a very strong reason to be an exception to "preserve" the definite article (as Odyssey is). -DePiep (talk) 10:56, 13 March 2019 (UTC)
- And in this case, the symbol Ge is definite, even if it has not been previously been specified, so the article should be there. Our article on The also mentions in its first sentence: "The ... is a grammatical article in English, denoting persons or things already mentioned, under discussion [my emphasis], implied or otherwise presumed familiar to listeners or readers", and since the symbol (again) is under discussion, the article should again be there. Double sharp (talk) 13:47, 8 March 2019 (UTC)
- It is not about whether "Ge" is a symbol of sorts. It is about using the definite article "The" here. From the article: '... denoting thing(s) already mentioned' while that is exactly not the case in the opening sentence: it is introducing the terms. See also Merriam Webster Online Dictionary.: [The] — used as a function word to indicate that a following noun or noun equivalent is definite or has been previously specified by context or by circumstance - DePiep (talk) 10:57, 2 March 2019 (UTC)
- Support per YBG. And btw. "the" has been added a lot of times to the element articles by many different users. but been reverted by one user only. Christian75 (talk) 05:28, 1 March 2019 (UTC)
- Oppose because "with the symbol X and atomic number N" sounds even more clumsy than what we now have "with symbol X and atomic number N". Rather than cluttering up these WP:NOTVOTEs with a long list of possibilities, I will add a menu of choices elsewhere. YBG (talk) 07:10, 1 March 2019 (UTC)
Additional alternatives
|
The above discussion is considering only two choices, but there are many others; listed here using carbon.
|
#1(a) - #10(a) were added later YBG (talk) 15:15, 1 March 2019 (UTC)
|
Of all of these, I prefer #10. It has the fewest words (though not characters). It uses "the" to show that it is the one and only such element. It clearly separates the atomic number (which is defining) and the symbol (which is merely descriptive). It uses the WP convention of bolding synonyms. But I think several others are improvements over the current text (1) and the current proposal (2). Thoughts, anyone? YBG (talk) 07:31, 1 March 2019 (UTC)
- I agree that #10 is the best. --Khajidha (talk) 12:20, 1 March 2019 (UTC)
- To be complete: why not write "with the atomic number 6"? -DePiep (talk) 14:10, 1 March 2019 (UTC)
- @DePiep: OK, I've added additional options #11-20, but I don't think it changes which one I think is best, nor, I suspect, Khajidah. YBG (talk) 15:15, 1 March 2019 (UTC)
- To be complete: why not write "with the atomic number 6"? -DePiep (talk) 14:10, 1 March 2019 (UTC)
- Maybe my question could be taken literally? Why did no one not have 'natural' impulse to include the "The number" in this discussion? (My guess: because it does not feel that natural, for a reason). -DePiep (talk) 11:42, 2 March 2019 (UTC)
- I agree with you that no one suggested that we add "the" before "atomic number" because it does not feel natural, and that it does not feel natural for a good reason. My list of all the options with "the" added before "atomic number" serves to highlight this unnaturalness, and may be instructive to others. Or maybe it will only serve to illustrate my OCD tendencies. YBG (talk) 19:13, 2 March 2019 (UTC)
- Maybe my question could be taken literally? Why did no one not have 'natural' impulse to include the "The number" in this discussion? (My guess: because it does not feel that natural, for a reason). -DePiep (talk) 11:42, 2 March 2019 (UTC)
Opening sentence more friendly
The opening sentences of our ledes for the elements are rather unfriendly, having too much of a focus on technical details (which are duplicated in any event, in the side bar). Take the lede paragraph for zirconium:
Zirconium is a chemical element with symbol Zr and atomic number 40. The name zirconium is taken from the name of the mineral zircon (the word is related to Persian zargun (zircon;zar-gun, "gold-like" or "as gold")), the most important source of zirconium.[1] It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, three of which are stable. Zirconium compounds have no known biological role.
Compare with the entry in the OED:
"A metallic element, obtained from zircon as a black powder or as a greyish crystalline substance. Symbol Zr."
Now have a look at this rewrite:
Zirconium is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Its name is taken from the name of the mineral zircon (the word is related to Persian zargun (zircon;zar-gun, "gold-like" or "as gold")), the most important source of zirconium.[2] It is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. It forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Zirconium is the 40th member of the periodic table of chemical elements, and is denoted by the symbol Zr.
Which option is more friendly for the general reader? Sandbh (talk) 00:03, 2 March 2019 (UTC)
- For starters, we could write the symbol as "Zirconium (Zr)". Does this improve friendly reading? Ans: is it correct (bold it)? -DePiep (talk) 11:34, 2 March 2019 (UTC)
- My initial reaction was not positive, but it has grown on me. I still think the atomic number should figure a bit more prominently. What about moving some or all of the last sentence closer to the front, like this:
- Zirconium (Zr) is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. It is the 40th member of the periodic table of chemical elements.
- Or
- Zirconium (Zr) is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. It the chemical element with atomic number 40.
- But if I give it a while longer, the idea of having the standardized, same-format-for-all-elements sentence at the end of the lede paragraph may also grow on me. YBG (talk) 19:28, 2 March 2019 (UTC)
- I can support going to the "Zirconium (Zr)" opening (still wondering if the symbol should be bold). Moving the atomic number further away from opening (as YBG illustrated) is positive, because it allows for opening with a strong, readible sentence, highlighting main properties and reducing jargon & code. I reject this idea: "Zirconium (Zr, atomic number 40)", because too much bracketed text (glad we got rid of the pronuncation soup there). Still, the notion "chemical element" (as opposed to compound) is required, and in top. Comes to mind that the link chemical element is not in the infobox?!
- For the same reason 'don't clutter the sentence', I'd say do not bold the symbol. -DePiep (talk) 11:07, 13 March 2019 (UTC)
- My initial reaction was not positive, but it has grown on me. I still think the atomic number should figure a bit more prominently. What about moving some or all of the last sentence closer to the front, like this:
- For starters, we could write the symbol as "Zirconium (Zr)". Does this improve friendly reading? Ans: is it correct (bold it)? -DePiep (talk) 11:34, 2 March 2019 (UTC)
-DePiep (talk) 11:07, 13 March 2019 (UTC)
- In the infobox, we change the sectionheader into "In the periodic table of chemical elements" (2 lines in header?). -DePiep (talk) 12:12, 13 March 2019 (UTC)
References
- ^ Harper, Douglas. "zircon". Online Etymology Dictionary.
- ^ Harper, Douglas. "zircon". Online Etymology Dictionary.
Proposal, concept
I want to develop a proposal. Here are my suggestions (numbers per 100, no further meaning):
- 100. "Zirconium (Zr)" -- Article opens with name in bold, then symbol in brackets, not bolded.
- 200. Opening sentence: Free. It best be strong, catching, well-written, highlighting the element (not: ...?). Readability.
- 300. "chemical element": this clarification is required in the opening sentence imo, because readers must be told this is not a compound (diff: water - gold - stone, somehow). So we need, like: "gold is a chemcal element". Can be/is this word+wikilink in the infobox?
- 400. Atomic number: I don't know. It's in the infobox. Need to mention this in opening section?
Questions:
- Q01: Reader's interest re gold, re seaborgium?
- Q02: Differentiate this proposal per set, like: He is not Sg?
- (A?: a more free first sentence can solve much of this).
- TBH, we could decide on #100 right away, but the "sentence" and readibility is also relevant. IMO. -DePiep (talk) 21:53, 13 March 2019 (UTC)
- OK then. I will propose and support the format being "Zirconium (Zr)". -DePiep (talk) 20:32, 30 March 2019 (UTC)
- TBH, we could decide on #100 right away, but the "sentence" and readibility is also relevant. IMO. -DePiep (talk) 21:53, 13 March 2019 (UTC)
Atomic weight of radioactive elements
For elements without a stable isotope, is it okay to use the isotopic mass instead of the atomic number of the longest-lived isotope for calculating molar masses? Either way, which isotope should I use for technetium? (The two longest-lived isotopes have half-lives that are very close to each other.) Care to differ or discuss with me? The Nth User 23:41, 13 March 2019 (UTC)
- Asking for a friend: see highly useful Template:Chem molar mass(edit talk links history) development. -DePiep (talk) 23:43, 13 March 2019 (UTC)
- In a case where you have the actual element, you presumably know which isotope you have of it, so you use that to calculate the molar mass. By default I'd suggest using the most commonly produced isotope, in this case 99, even if it happens to not be the most stable. Double sharp (talk) 05:10, 8 April 2019 (UTC)
Origin of name "flerovium"
The article needs to explain the origin of this official name. See Talk: Isotopes of flerovium#Origin of name?. --Thnidu (talk) 02:33, 8 April 2019 (UTC)
- @Thnidu: It was already explained at the main article on the element at Flerovium#Naming. Double sharp (talk) 05:08, 8 April 2019 (UTC)
- @Double sharp: Thank you. --Thnidu (talk) 11:22, 8 April 2019 (UTC)
MAX phase(s)
Should MAX phases be moved to MAX phase? This would be consistent with WP:SINGULAR, but there may be extenuating circumstances. YBG (talk) 02:26, 23 September 2018 (UTC)
- See also WP:PLURAL. YBG (talk) 02:30, 23 September 2018 (UTC)
- Singular. It is a list at best, not a class. And IMO no other plural-rules apply. -DePiep (talk) 07:07, 23 September 2018 (UTC)
- But why is it named "Phases" at all? DePiep (talk) 23:51, 23 September 2018 (UTC)
- Good point. Phase lists many definitions, including
Phase (matter), a physically distinctive form of a substance, such as the solid, liquid, and gaseous states of ordinary matter—also referred to as a "macroscopic state"
. wikt:phase saysA component in a material system that is distinguished by chemical composition and/or physical state (solid, liquid or gas) and/or crystal structure. It is delineated from an adjoining phase by an abrupt change in one or more of those conditions
. But the article, while explaining MAX in detail nowhere explains "phase". Can anyone check the literature to come up with an explanation? And perhaps decide what is the best article? From a linguistic point of view, "MAX phase alloy" seems much better than either MAX phases or MAX phase, but I defer to others. YBG (talk) 17:30, 29 September 2018 (UTC)
- Good point. Phase lists many definitions, including
Having re-read WP:PLURAL, I now propose to move the article to MAX phase alloys. It seems more a class than a list. YBG (talk) 22:07, 11 December 2018 (UTC)
Categories into plural?
- Reading WP:PLURAL especially exception #1 re Class (set theory) and further orthography, I think we should
re-examplere-examine our our category names: "Alkali metals"? (article title, periodic table legend). -DePiep (talk) 05:43, 12 October 2018 (UTC)- As I read WP:PLURAL, it seams obvious that our category articles should be plural. These articles seem to fit WP:PLURAL so well that they could well serve as additional bulleted examples. YBG (talk) 07:07, 12 October 2018 (UTC)
- I agree. If none disagrees, please start a Requested Move discussion for these categories.--R8R (talk) 13:57, 12 October 2018 (UTC)
- Let's flesh it out a bit first. See consequences, check similar/different situations. -DePiep (talk) 15:55, 12 October 2018 (UTC)
- I agree. If none disagrees, please start a Requested Move discussion for these categories.--R8R (talk) 13:57, 12 October 2018 (UTC)
- As I read WP:PLURAL, it seams obvious that our category articles should be plural. These articles seem to fit WP:PLURAL so well that they could well serve as additional bulleted examples. YBG (talk) 07:07, 12 October 2018 (UTC)
- Would imply Metal → Metals. -DePiep (talk) 22:08, 13 October 2018 (UTC)
- I doubt it. While, for example, alkali metals are clearly first and foremost a specific set of elements that often go together, this is not the case with metals, which is first and foremost rather a singular term that applies to many elements. The word "metal" simply has too much value on its own to be considered primarily as a subset of the chemical elements.
- I think we should pluralize titles of our sets of elements that we use in our main PT (alkali metal, post-transition metal, etc.) and alike categories that don't fit into that scheme (platinum group metals, pnictogens, etc.) as well as group and period articles (group 3 element, period 6 element, etc.)--R8R (talk) 12:14, 14 October 2018 (UTC)
- I mentioneed "metal" because it is in the same PT legend, only one class higher. That is, if we apply class concepts & naming to alkali metals etc., we should also appy it to the higher class. Wrt "platinum group metals" &tc.: probably yes, sure let's get that list together (from here?). wrt group 3 element: never into "group 3 elements", change it into "group 3". PT groups already have a name, no need to create a detour. "FC Barcelona" is the club, not "FC Barcelona players" (IOW, don't define a class by listing its members, but do so by describing/naming the class). See the 2013 disappointment. In general, we need to apply the "class" concept thoroughly (as applied by linguists, mathematicians, ...). -DePiep (talk) 18:18, 14 October 2018 (UTC)
- Metal is used not only to name a class of ~95 chemical elements, but also for innumerable alloys and other compounds. In common speech, it refers to a generic type of material, not to a specific class, comparable to plastic, wood or paper. Like soap and glass, it has both a common definition and a technical chemical definition. But our category names and the group names refer to definite finite classes of elements, and so IMHO should be treated differently. I would have our PT legend say "[[alkali metals]]" but "[[metal]]s" for consistency. YBG (talk) 20:54, 14 October 2018 (UTC)
- (re last sentence:
I think you meant to write the opposite?) Sounds good. Still, if the article stays "metal", we can label it "metals" in the PT legend. SAme for "non-metal/s" I guess. What with article "metalloid/s"? -DePiep (talk) 04:57, 15 October 2018 (UTC)
- (re last sentence:
- Metal is used not only to name a class of ~95 chemical elements, but also for innumerable alloys and other compounds. In common speech, it refers to a generic type of material, not to a specific class, comparable to plastic, wood or paper. Like soap and glass, it has both a common definition and a technical chemical definition. But our category names and the group names refer to definite finite classes of elements, and so IMHO should be treated differently. I would have our PT legend say "[[alkali metals]]" but "[[metal]]s" for consistency. YBG (talk) 20:54, 14 October 2018 (UTC)
- I mentioneed "metal" because it is in the same PT legend, only one class higher. That is, if we apply class concepts & naming to alkali metals etc., we should also appy it to the higher class. Wrt "platinum group metals" &tc.: probably yes, sure let's get that list together (from here?). wrt group 3 element: never into "group 3 elements", change it into "group 3". PT groups already have a name, no need to create a detour. "FC Barcelona" is the club, not "FC Barcelona players" (IOW, don't define a class by listing its members, but do so by describing/naming the class). See the 2013 disappointment. In general, we need to apply the "class" concept thoroughly (as applied by linguists, mathematicians, ...). -DePiep (talk) 18:18, 14 October 2018 (UTC)
- Would imply Metal → Metals. -DePiep (talk) 22:08, 13 October 2018 (UTC)
@DePiep: No, I think I said it as I intended. Here is what I'd see as the first part of our PT footer legend:
| [[metal]]s | [[metalloids]]? | [[nonmetals]]? | ||||||
| [[Alkali metals]] | [[Alkaline earth metals]] | [[Lanthanides]] | [[Actinides]] | [[Transition metals]] | [[Post-transition metals]] | [[Reactive nonmetals]] | [[Noble gases]] | |
This has the metal article title in the singular but all other category and super-category article titles in the plural, although ? indicates I'm a bigbit unsure about "metalloid" and "nonmetal". YBG (talk) 05:53, 15 October 2018 (UTC)
- OK, it was about the wikilabels etc. We understand that the plural article pages do have the content, not redirect. -DePiep (talk) 15:23, 15 October 2018 (UTC)
- Yea, I was trying to indicate by the wikilinks which article would be the real article with content. YBG (talk) 17:51, 15 October 2018 (UTC)
- OK, it was about the wikilabels etc. We understand that the plural article pages do have the content, not redirect. -DePiep (talk) 15:23, 15 October 2018 (UTC)
- re the ?-question marks remaining: better [[metalloids]] as article (because the chemical class is not main issue), and treat nonmetals like metals: [[nonmetal]]s because similar to "metal" more general meaing. -DePiep (talk) 00:35, 21 October 2018 (UTC)
- OK, I'll buy in to [[metalloids]]. But I would decide [[nonmetal]]s/[[nonmetals]] based on the article content because:
- An article titled [[nonmetals]] seems like it would describe a definite class rather than a type of substance, so I would expect to see an article about a definite class of 17± specific chemical elements.
- An article titled [[nonmetal]] seems like it would describe a type of substance rather than a definite class, so I would expect to see not only about those specific chemical elements, but also about substances commonly referred to as non metal like paper, wood, glass, and the like, as in the sign I described at User talk:YBG/Archive 4 § Re nonmetals
- It is a subtle distinction, but one reinforced by the article metal which describes not a (just) specific definite class of chemical elements but rather a general type of substance; not just metallic chemical elements, but also alloys and other "metal" substances in the common vernacular. But maybe this is too subtle. Thoughts? YBG (talk) 08:36, 21 October 2018 (UTC)
- (do not archive) -DePiep (talk) 15:13, 13 November 2018 (UTC)
- While not in our standard legend, rare-earth metal/s might qulify for plural for the same reason. Todo: check other set names in Names for sets of chemical elements. -DePiep (talk) 15:17, 13 November 2018 (UTC)
- So YBG, following your clear legend illustration above: we want article titles (=pages with the content) in plural for our categories, but not for top categories (metal, nonmetal, and therefor metalloid). That's eight to be plural (AMs -- NGs). We better not introduce other name changes, I strongly suggest. Now how to proceed? Strangely, WP:MOVE is not clear. I guess we need to start at Talk:Alkali metal. Anyway, shall we go on and spend serious time on this? I'd like it to be done convincingly crisp & clean. -DePiep (talk) 21:22, 15 December 2018 (UTC)
- OK, I'll buy in to [[metalloids]]. But I would decide [[nonmetal]]s/[[nonmetals]] based on the article content because:
- (do not archive for now) -DePiep (talk) 21:34, 24 January 2019 (UTC)
Categories into plural: step 1
I am preparing a proposal to change these article names into plural (so that Alkali metals has the content). However, I found this thing we should solve first. Currently, the names in the legend are singular! That does not support the wish to make them plural:
I propose to make these texts show as plural in the legend (use labels: [[Alkali metal|Alkali metals]]). Reasoning re WP:PLURAL and class is in step 2, below (=in the actual proposal). YBG
- !votes? -DePiep (talk) 17:49, 17 February 2019 (UTC)
- support. Excellent starting point for this change. As usual, @DePiep:, your attention to detail serves our project well. YBG (talk) 06:06, 18 February 2019 (UTC)
- Comment: re plural usage: when meaning a class, wouldn't that imply we should take a look at articles text like nonmetal and infoboxes: plural sectionheaders in there? Is this a WP:ELEMENTS guideline ahead? -DePiep (talk) 06:04, 19 February 2019 (UTC)
- Support. Indeed a good place to start; I find more often than not that we speak of element categories as a whole, which does imply WP:PLURAL. ComplexRational (talk) 14:10, 19 February 2019 (UTC)
- Support I've read WP:PLURAL a few times and arrived to the conclusion this is indeed the sort of thing plural forms in titles are reserved for.--R8R (talk) 15:23, 19 February 2019 (UTC)
- Asking Sandbh, Double sharp could you chime in? You've been working extensively with this in articles. -DePiep (talk) 19:04, 19 February 2019 (UTC)
- Comment Plural for article names is fine. Plural for legend names is odd. When I see Na in red, and look up the legend, I see that Na is an alkali metal. It is not an "alkali metals". Sandbh (talk) 23:42, 19 February 2019 (UTC)
- That's the point, albeit inversed. What you see here is: Na is in the class of alkali metals, good. The class is defined by itself, not by listing its elements. (Not: "FC Barcelona team is these eleven players", but "These eleven players are in the FC Barcelona team"). The class is not defined by its member list.
- Sure one can write & link: "Sodium is an alkali metal", still then the article starts with: "The alkali metals are a ...". The class is the predominant meaning, and so it is used in the legend. And sure, plurals in the legend do not break the reading or intention, it's about one's understanding.
- Please take another look at WP:PLURAL and class (set theory) definitions to get the orthography of our category classification. -DePiep (talk) 07:46, 20 February 2019 (UTC)
- (edit conflict) I wouldn't have a problem with plural legends. If I see Na in red, and look up the legend, I see that Na is one of the alkali metals. Just like if the legend is in the singular, I don't look at the legend and say, Oh, the red swath is an alkali metal; no, I'd say, Oh, each red cell is an alkali metal. I think singular legend and plural legend both work OK. But I'd prefer to have the legend correspond to the article title. So currently, I prefer the legend to be singular, but if we change the article title to plural, I'd prefer the legend to be plural. YBG (talk) 07:51, 20 February 2019 (UTC)
- Asking Sandbh to take an other check. Yes, as you describe the singular can be used in the legend, lingustically correct. However, the proposal is to use plural for the same reason their articles better be plural: it is the name of a class, linguistically correct too of course. Also, please specify how strong you oppose this: block it, or is the change acceptable for you ;-) ? (Problem is that we'd have a contradiction when concluding "plural" for the article but "singular" when actually used as a class name). -DePiep (talk) 10:47, 26 February 2019 (UTC)
- Just a note that deciding to use the plural form to name the article about the class does not prohibit us from using the singular for a member of the class. However, IMO in high-profile situations (like legends) where either singular or plural could be reasonably used, it seems to me we are better off being consistent with the article titles. But I will bow to any strongly held opinion to the contrary. YBG (talk) 17:06, 26 February 2019 (UTC)
- Waiting here for Sandbh, thats all. -DePiep (talk) 23:15, 28 February 2019 (UTC)
- Just a note that deciding to use the plural form to name the article about the class does not prohibit us from using the singular for a member of the class. However, IMO in high-profile situations (like legends) where either singular or plural could be reasonably used, it seems to me we are better off being consistent with the article titles. But I will bow to any strongly held opinion to the contrary. YBG (talk) 17:06, 26 February 2019 (UTC)
- Asking Sandbh to take an other check. Yes, as you describe the singular can be used in the legend, lingustically correct. However, the proposal is to use plural for the same reason their articles better be plural: it is the name of a class, linguistically correct too of course. Also, please specify how strong you oppose this: block it, or is the change acceptable for you ;-) ? (Problem is that we'd have a contradiction when concluding "plural" for the article but "singular" when actually used as a class name). -DePiep (talk) 10:47, 26 February 2019 (UTC)
- (edit conflict) I wouldn't have a problem with plural legends. If I see Na in red, and look up the legend, I see that Na is one of the alkali metals. Just like if the legend is in the singular, I don't look at the legend and say, Oh, the red swath is an alkali metal; no, I'd say, Oh, each red cell is an alkali metal. I think singular legend and plural legend both work OK. But I'd prefer to have the legend correspond to the article title. So currently, I prefer the legend to be singular, but if we change the article title to plural, I'd prefer the legend to be plural. YBG (talk) 07:51, 20 February 2019 (UTC)
- Support Sandbh (talk) 11:11, 1 March 2019 (UTC)
Categories into plural: step 2
After step 1, we can propose to change the article titles. That proposal (step 2) is in my User:DePiep/sandbox2.
We can discuss the sandbox preparation here (do not !vote the proposal itself now). I want a non-problematic change, so it must be convincingly strong right away (unlike failed attempt like this, six years of linguistical pain). YBG -DePiep (talk) 17:49, 17 February 2019 (UTC)
Step 3: Formal Move proposal
- See Talk:Alkali_metal#Requested_move_28_February_2019. -DePiep (talk) 22:31, 23 March 2019 (UTC)
- Unfortunately, it was closed as "no consensus" without further elaboration. My impression was that thos involved (WP:ELEM, WP:CHEMISTRY) had a better grasp and lesser need to invoke parallels-or-not (dog/dogs). -DePiep (talk) 08:55, 12 April 2019 (UTC)
Article from Science mag about the current outlook on making superheavies
Here. (They also linked to this old one from 2012 when RIKEN got its third atom of Nh.) Double sharp (talk) 07:58, 12 April 2019 (UTC)
- The SHE Factory is ready and the new accelerator was launched last December; this month it will be tested by redoing the 48Ca experiments to synthesise Fl and Mc. Double sharp (talk) 10:23, 12 April 2019 (UTC)
Nomination for deletion of Template:Periodic table (p-block trend)
![]() Template:Periodic table (p-block trend) has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. -DePiep (talk) 19:30, 27 April 2019 (UTC)
Template:Periodic table (p-block trend) has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. -DePiep (talk) 19:30, 27 April 2019 (UTC)
colour/property edits to Elements articles
Hi, I'm assuming good faith, but a number of edits have been made to different element related articles by "Is Pepsi Ok?". At first glance there is nothing particularly wrong with these edits, but then specific changes are being made to our description of the elements which may not be as accurate as the original wording and such changes should be accompanied by citations to reliable sources. I don't want to discourage a good faith editor by reverting all their contributions so have brought it here for discussion. Cheers Polyamorph (talk) 05:44, 17 April 2019 (UTC)
- "Is Pepsi Ok?" is Sethrc225 (talk · contribs) somehow. -DePiep (talk) 16:09, 17 April 2019 (UTC)
- They have been notified/warned: User_talk:Sethrc225#April_2019_2. -DePiep (talk) 16:11, 17 April 2019 (UTC)
Thank you, I am trying to go back and add citations to all these claims at the moment. Thank you for the notice Sethrc225 (talk) 18:48, 17 April 2019 (UTC)Sethrc225
- Thanks Sethrc225, and can I again stress to others that we should not be discouraging new editors, so have some patience while they learn the ropes please. Polyamorph (talk) 16:58, 18 April 2019 (UTC)
Daily Bruin article
A short item about the various layouts of the table, based on interviews with Eric Scerri, Philip Stewart, and myself.
It has a couple of shortcomings: "orientation" instead of "organization"; and an upside down Janet table, with He in two places, sort of. Otherwise interesting. Sandbh (talk) 05:30, 13 April 2019 (UTC)
- Re the second image in short item:
- Please tell Eric Scerri, Philip Stewart, User:Sandbh: do not ever use an 18-column periodic table again. (It has caused enough trouble, as they all know). Wheatever you want to say: say it in 32-columns (>=32).
- Yes, I understand the Eric and Philip both support a 32-column form. Sandbh (talk) 10:40, 14 April 2019 (UTC)
- Also, someone could ask Pyykkö to solve & clarify group 3 in their description before going about extensions. -DePiep (talk) 20:02, 13 April 2019 (UTC)
- Added "please" to change tone. -DePiep (talk) 09:34, 21 April 2019 (UTC)
Detection of HeH+ in the interstellar medium
https://www.nature.com/articles/s41586-019-1090-x
First compound in the universe. Helium acting as a metal. Extraordinary! Lends support to He over Be, with He coloured as a noble gas. Sandbh (talk) 10:56, 18 April 2019 (UTC)
- HeH+ was known long ago, just only in the laboratory. Double sharp (talk) 17:22, 18 April 2019 (UTC)
- Quite so. I was struck by the contrast between a laboratory curiosity and the cosmological beginning of chemistry. Sandbh (talk) 00:54, 19 April 2019 (UTC)
- Certainly; though when you have nothing but H and He around there's not much choice, it is really striking that H2 came later than HeH+! Incidentally, since EN(H) < EN(He) I am not sure this counts as He acting as a metal. Double sharp (talk) 12:15, 20 April 2019 (UTC)
- Hmm. I found this paper:
- Certainly; though when you have nothing but H and He around there's not much choice, it is really striking that H2 came later than HeH+! Incidentally, since EN(H) < EN(He) I am not sure this counts as He acting as a metal. Double sharp (talk) 12:15, 20 April 2019 (UTC)
- Quite so. I was struck by the contrast between a laboratory curiosity and the cosmological beginning of chemistry. Sandbh (talk) 00:54, 19 April 2019 (UTC)
- "The dipole moment sign for HeH+ (A- B+) molecule indicates that H atom is more positive that He. It is in agreement with eletronegativity arguments. The results from Mulliken populations shown that the charge on He atom is +0.47 and on H atom is +0.53. The value of dipole moment is in good agreement with GAMESS theoretical results for a STO-3G basis functions."
- Presumably the greater nuclear charge of He would also mean that the two electrons would spend more of their time near its nucleus?
- Will that do? Sandbh (talk) 01:54, 21 April 2019 (UTC)
- Yes, He with its greater valence electron density and filled shell is more electronegative than H. (Similarly Ne is the most electronegative of all elements, as it beats He soundly in valence electron density.) I have a sortable table showing electronegativity values used in Droog Andrey's periodic table poster in my userspace (with his permission); there He is 4.20 and Ne is 4.50, both beating F at 4.00. Double sharp (talk) 20:33, 21 April 2019 (UTC)
- Will that do? Sandbh (talk) 01:54, 21 April 2019 (UTC)
- Unrelated? Today, Main page has
Scientists announce the discovery of naturally occurring helium hydride ions, believed to be the first compound to have formed in the universe, in the planetary nebula NGC 7027
- DePiep (talk) 23:50, 21 April 2019 (UTC)
- Yup, that's it! Double sharp (talk) 05:12, 22 April 2019 (UTC)
Another look
At the start of the Nature letter the authors say:
- "In this metal-free and low-density environment, neutral helium atoms formed the Universe’s first molecular bond in the helium hydride ion HeH+ through radiative association with protons."
However, the first reaction pathway they list (p. 357) is: He+ + H → HeH+ + hv [1]
Later they write: "…the reaction He + H+ → HeH+ + hv [2]…dominates HeH+ formation in the early Universe…" and repeat this assertion later on the same page (p. 358).
So, in reaction [1] helium acts as a nonmetal i.e. it gains an electron whereas in [2] it reacts as a metal i.e. it loses an electron.
The authors aren’t clear on this point but my interpretation of their letter is that [2] no longer predominates.
Be that as it may, it's remarkable that (like H), helium is capable of reacting either like a metal or a nonmetal. Sandbh (talk) 10:04, 24 April 2019 (UTC)
Mendeleev's "cubic" system


@R8R: could you please look up one of Mendeleev’s articles? The citation is Zhurnal Russkoe Fiziko-Khimicheskoe Obshchestvo, 1869, 1, 60–77. The article may be "On the correlation between the properties of the elements and their atomic weight".
The bit I’m interested in is note 2, where Mendeleev says in part, "It appears to me that the most natural approach would be to construct a cubic system (the one recommended is exactly this). However, the attempts at such construction have not led to any real results." This quote is from Jensen’s 2005 book, Mendeleev on the periodic law: Selected writings, 1869–1905. It comprises English translations of German translations of the original Russian sources.
Nobody seems to know what to make of the subject passage in Note 2, which makes me suspect the translation was bad.
The question is, what did Mendeleev have in mind when he referred to a "cubic" system? The one he recommends in his article has nothing cubic about it. It is the same as the flat one published in 1869, in Zietschrift für Chemie, 12, 405—406.
In 1911, van den Broek attempted to design a "cubic" system, based on Mendeleev's note 2.
According to van den Broek his system was "[a] cubic system, consisting of five major periods, three small periods of 8 elements, and therefore a cube fives places high, three places deep and eight places wide, with 120 locations". In each case, the elements shown diagonally are those that are supposed to represented along the third dimension.
thank you, Sandbh (talk) 02:22, 17 March 2019 (UTC)
- Hello. I have some comments:
- Mendeleev in your quote says that "the attempts at such construction have not led to any real results." Given that, it rather makes sense, it makes sense he did not publish a cubic periodic table because he was unable to find to construct one.
- An English translation of your article is freely available on the Internet. After reading his explanation (note 2 in the end of the article), I was unable to figure what kind of a cubic periodic table he had in mind (but maybe you can understand his line of thought?). He did not provide a sketch of what that would look like because, as I mentioned, he was unable to formulate what it should have looked like.
- I was unable to look up a Russian original version of the article. It may be very well be possible, but I decided not to try too hard after running into initial difficulties in presumption the English translation would be enough anyway.--R8R (talk) 12:03, 17 March 2019 (UTC)
- In the van den Broek table, why does it look like there are additional "unknown elements" not included in Mendeleev's table and certainly nonexistent in our periodic table? For example, there are three blank spaces between copper and zinc in columns IV–VI. Also, regarding atomic weights, what is meant by 52 for iron, 54 for cobalt, 56 for nickel, etc.? I am not quite sure what these spaces and values are supposed to represent, as I have never seen them in any other periodic table. Might this be related to the statement that "attempts at construction have not led to any real results"? ComplexRational (talk) 12:47, 17 March 2019 (UTC)
- Van den Broek theorised that the system of elements contained exclusively triads. So he had to presume lots of unknown elements, even though he had already made radioactive decay products fill many vacant spaces. These were not new elements in any case. The 52, 54, and 56 etc are the atomic numbers x2. Sandbh (talk) 05:13, 21 March 2019 (UTC)
- In the van den Broek table, why does it look like there are additional "unknown elements" not included in Mendeleev's table and certainly nonexistent in our periodic table? For example, there are three blank spaces between copper and zinc in columns IV–VI. Also, regarding atomic weights, what is meant by 52 for iron, 54 for cobalt, 56 for nickel, etc.? I am not quite sure what these spaces and values are supposed to represent, as I have never seen them in any other periodic table. Might this be related to the statement that "attempts at construction have not led to any real results"? ComplexRational (talk) 12:47, 17 March 2019 (UTC)
@R8R: The question is, why did Mendeleev say, "the one recommended is exactly this" i.e. cubic, when it clearly isn't? The English translation is a translation of the German translation of the Russian original. I have read that some translations of Mendeleev's works suffered from mistakes in translation. Given the confusing nature of Mendeleev's comments, and the fact that the English version is a translation of a translation I firmly suspect the problem lies in the translation of the original Russian. Hence my request. Sandbh (talk) 06:59, 18 March 2019 (UTC)
- Ah, I see. Will do.--R8R (talk) 15:42, 18 March 2019 (UTC)
- I was finally able to find a reprint of the said article. Indeed, you were correct in suspecting that the parenthesized note was not properly translated. The quote you listed at the top of this section would be closely translated to "It seems most natural to me to construct a cubic system (the one being suggested is plane-based), but attempts for its formation have also not led to any proper results" (p. 13). His phrasings a little bit off for me as a Russian speaker, so it's not just my poor translating skills :) --R8R (talk) 10:47, 19 March 2019 (UTC)
- Wow! One word mistranslated in going from Russian to German, and the whole meaning changes, confusingly. Thank you very much R8R. Sandbh (talk) 05:03, 21 March 2019 (UTC)
- I'm sorry it took me so long to reply. You are very welcome.--R8R (talk) 12:20, 1 May 2019 (UTC)
- Wow! One word mistranslated in going from Russian to German, and the whole meaning changes, confusingly. Thank you very much R8R. Sandbh (talk) 05:03, 21 March 2019 (UTC)
- I was finally able to find a reprint of the said article. Indeed, you were correct in suspecting that the parenthesized note was not properly translated. The quote you listed at the top of this section would be closely translated to "It seems most natural to me to construct a cubic system (the one being suggested is plane-based), but attempts for its formation have also not led to any proper results" (p. 13). His phrasings a little bit off for me as a Russian speaker, so it's not just my poor translating skills :) --R8R (talk) 10:47, 19 March 2019 (UTC)
IUPAC and Group 3

Here's a 32-column table which aims to resolve the La v Lu question, through a synthesis or rapprochement of each camp.
Features
- Helium colour coded as a noble gas;
- No need for a split d-block, unless that's your preference;
- Idealised electron configurations are shown, as are modular blocks;
- Colour categories are my take on the chemistry involved; anyone can use their own colours and categories;
- Black and white shading to emphasise the contrast between alkaline metals and the corrosive non-metals;
- Rainbow shading to emphasise L-R progression;
- Grey shading for noble gases to emphasise their bridging role;
- Balanced 6-6-5-6 categorisation of the nonmetals;
- Category notes expand the modern chemistry theme of the table;
- Nice first row distinctiveness s >> p > d > f;
- Group 3 noted as bifurcating after Y into an La branch, and an Lu branch;
- Symmetry of the L-R progression in metallic to nonmetallic character is noted in Table 2;
- The p-block, with no less than six categories, is Diversity Central;
- Can be deconstructed into a Janet form, or a tetrahedral table in a cube;
- Whole thing, including category notes, can be rearranged into a medium long table;
- No conflict with IUPAC form;
- Anomalous configurations listed;
- Prior La-Ac tables remain valid;
- Consistent with Scerri's views that La and Lu cannot be resolved by appeal to physical or chemical properties;
- Not inconsistent with old school treatments of group 3 as including the Ln and An;
- Consistent with the notion of inter-block bridging groups that show properties in between, or that are a mixture of, groups to either side: group 3; groups 11-12; and group 18; and
- A chemistry book chapter on this group 3 would make fascinating reading (a good thing given, to date, that group 3 is supposed to be the least studied group).
Observations
Note the distinction between the 14 element wide f block, and the 30 elements of the Ln and An.
The black shading of the pre-transition metals can take some getting used to. Even so, I couldn’t go past the beauty of the black and white analogy.
While I feel the split-d block version offers more richness this is no longer a show-stopper for me.
I'm not suggesting we adopt this table. I've posted it here only in the context of the IUPAC project on the composition of group 3.
@Double sharp: @R8R: @Droog Andrey: @DePiep: I hope you like it.
How does it look to everyone? Sandbh (talk) 03:12, 9 April 2019 (UTC)
BTW Over five years ago, Double sharp began to feel that Group 3 bifurcates into -La-Ac, and -Lu-Lr. Sandbh (talk) 05:28, 9 April 2019 (UTC)
- My ideal table would look something like this indeed, well maybe except that I'd keep He over Ne. ^_^ I also mentioned the group II bifurcation there (-Ca-Sr-Ba vs -Zn-Cd-Hg), so maybe we can think about resurrecting the old A-B numbering too. ^_^ Double sharp (talk) 05:50, 9 April 2019 (UTC)
- Bifurcation, wow! Did not see that one coming. Looks like a wonderful scientific statement (description), and showing effective and elegant, this way. -DePiep (talk) 15:12, 9 April 2019 (UTC)
- Precisely! Thank you DePiep. Sandbh (talk) 02:33, 10 April 2019 (UTC)
- Bifurcation, wow! Did not see that one coming. Looks like a wonderful scientific statement (description), and showing effective and elegant, this way. -DePiep (talk) 15:12, 9 April 2019 (UTC)
I'd like to write some criticisms, since nobody else challenged this proposal. In advance, I'd like to say all of my future comments are written in good spirit and I most certainly welcome any further attempts to resolve as this at the very list gives us more options to choose from. Also, happy International Workers' Day.
- I seriously doubt that helium located over beryllium, even if color-coded as a noble gas, is an idea that will get wide approval among chemists.
- In principle, it does not seem all that bad an idea to have groups 3f and 3d (or 3-I/3-II, or whatever). This idea is certainly worth a wider discussion.
- The cropped version of the continuity of transition metals is rather strange and artificial. This is certainly not what I'm used to thinking of as of transition metals. I tend to think this skepticism will be shared by other people. Perhaps your idea would benefit from invention of a new term for what you have in groups 4--10/11 if these borders are indeed to stay this way.
- In general, I think coloring is rather secondary to what groups are to be like. I doubt that the current monochrome IUPAC table will suddenly come to full flower and color, not in the least because this may seem as a distraction from the group 3 question that is to be solved.
- Silberberg's table is fine if used as an inspiration, but I sincerely hope it won't be the final solution. If it is, then blocks need to be this crooked to work, and if they are, then we get a good hint we've come to a good limit of what a block is worth and raises the question of whether blocks are really needed if this is what they are.
Again, I do think that the 3f-3d idea is indeed worth a discussion. Kudos to you for coming up with it.--R8R (talk) 13:00, 1 May 2019 (UTC)
- No big deal about He. It can easily be moved over Ne. Eric's given up on He over Be.
- The colour categories are my take on the chemistry involved; anyone can use their own colours and categories.
- The blocks are still relevant in teaching the idealised form of the table. Once someone understands the idealised form then they can see what's really going on.
- Thanks for your recognition of the 3f-3d idea. Here's hoping it unblocks the 61-year old log jam. Landau and Lifshitz (1958, pp. 256–257) may have been the first to argue for Lu in group 3, in their case, on the basis of its complete 4f shell. Sandbh (talk) 07:22, 3 May 2019 (UTC)
- Landau LD & Lifshitz EM 1958, Quantum Mechanics (Non-relativistic Theory), Pergamon Press, Oxford
- I absolutely understand your reasoning re blocks. However, it rather explains why having blocks at all is a good idea (which it is if you don't constrain yourself with it) and not supports Silberberg's layout thereof. The point that I'm trying to make is that this is too complicated for an introduction into atomic orbitals. I think the -Lu-Lr layout I have always preferred would be ideal for the task, but even the split-d-block -La-Ac table is far better for this purpose than d elements atop f elements and some more d elements aside. At an introductory level, this is a rather complicated mess that is not going to sit well with many students anyway and it maybe even distorts the idea of periodicity itself.--R8R (talk) 11:33, 3 May 2019 (UTC)
18-column form

Here is the same periodic table in 18-column form. I do not want to promote any 18-col form (32-column form is much much clearer), but I created this one to prevent bad or plain wrong 18-col forms to appear.
It is a true cut-and-paste from the original 32-col form (as any good 18-col PT should be). No information was changed. I added "f-block" to the box below, as the word nicely and correctly fits there: it is the complete f-block. I choose the U+2606 ☆ WHITE STAR (☆) instead of asterisks to indicate "This is describing the 3-bifurcated structure" (sort of, just like we use 2-asterisks vertical aligned to indicate "group 3 = Sc/Y/La/Ac form"): a nonbinding indicator.
I note that we cannot use the words "lanthanides" and "actinides" below (as older PTs often do), because these two categories are cut and split in this PT. This absence is not a loss. At enwiki we abandoned this habit some time ago (for the same reason, plus that we consistently don't write other category names in there either).
When drawing the same PT but having group 3 "left" being Sc/Y/La/Ac and group 3 "right" being Lu/Lr (split d-block, as enwiki does today) which as Sandbh stated is equally correct for a bifurcated group 3, the bottom rectangle would be 58–71 and 90–103 (with the "3" above Lu/Lr), but would *not* be the f-block.
Again, drawing this group 3 issue (any group 3 issue!) in 18- not 32-column form does not help to clarify. For example, now there are two columns "3" ~below each other, and one must know (one cannot easily see) that these are not vertically aligned in the fork. Still, when doing so at least one should use the right basic PT. -DePiep (talk) 09:39, 12 April 2019 (UTC)
- Super!
- The "f-block" addition may be superfluous since there is an f-block flag below the f-block already.
- That's interesting about drawing this form in a split-d format. It probably doesn't matter that Lu/Lr would not be f-block, since in the current version La/Ac don't have any f-electrons either. You're going to have a discrepancy at one end of the f-block or the other. Since the block designations represent an idealised or pure form that doesn't exist in our universe, I may've been placing too much emphasis on the discrepancies as a way of guiding the group 3 question.
- The richness and beauty of the 32-column form is astonishing. Yes, if you turn it in an 18-column form it becomes harder to see straight away that the two group 3 columns aren't vertically aligned, as such. Then again many students find it hard to work out how the block really fits into the main body of the table, despite the asterisks or stars. Perhaps there should be a little "Aha!" inset somewhere on the same page showing how the blocks fit together. Sandbh (talk) 06:21, 13 April 2019 (UTC)
Silberberg's table

I was partly inspired by this image. Note the retention of the old school group numbering system, per Double sharp, and the identification of La-Ac as group 3B. ![]() Huzzah!
Huzzah!
Helium is correctly shaded as an s-block element.
The inset in the gap between the s- and d-elements shows the simplified or idealised filling sequence.
The periodic table shows the purported actual filling sequence.
Rather than renting the d-block asunder by moving Sc-Y- to the left, over La-Ac, group 3 has two branches after Y: an La-Ac branch, and an Lu-Lr branch. The principle of one group one place is retained. I know of no IUPAC convention or recommendation the prevents a group from having two branches as shown in this way.
La and Ac are rightly shown as d elements.
Depicting Lu and Lr as f elements rather than d elements is inconsistent with the depiction of helium, at the end of the 1s row, as an s-element. OTOH, if Lu and Lr are shown as d elements that would result in 42 d elements, two too many to be consistent with quantum mechanics. That said, if group 18 can have one s element and six p elements there is no reason why group 3 could not have four d elements, and two faux-f elements.
I think, however, that our expectations are unrealistic.
There is the idealised filling sequence exemplified by the Madelung Rule (the inset).
Then there is the real filling sequence (per my 32-column table).
We try to cobble these two ideas together which, in my experience, has always resulted in disharmony and confusion because every author I’ve read that takes this approach fails to fully explain what is going on.
Even Silberberg, as interesting as his table is, doesn’t quite get it right. At least he says:
- "Whenever our observations differ from our expectations, remember the fact always take precedence over the model; in other words the electrons don’t "care" what orbitals we think they should occupy." (p. 304)
Incidentally he treats the Ln as Ce to Lu.
By the time students get to the level at which this text is aimed at we can at least colour Lu and Th as d elements, and Lr as a p element, and then have an intelligent conversation about what’s going on. Sandbh (talk) 02:32, 10 April 2019 (UTC)
New notation form needed
In the Sandbh PT (2019-04-07), there now are two columns headed "3", depicting the single group 3 bifurcated. One column contains La, Ac, the other column contains Sc, Y, Lu, Lr.
It might be useful and needed to be able to differentiate between those two columns. For example, when the bifurcation is the topic (as it is in this PT, and likely in article Group 3).
- In history: I note that until recently, there always has been multi-columns with single group id: VIII has three columns (now 8, 9, 10).
- reuse A, B? Above, Double sharp suggests to reintroduce the A, B group notation (whichever form: CAS or European: an irrelevant difference in this), because of an other topic/discussion ("group 2 and 11 could be considered forked too"). First of all, that is a different discussion, and unclear, for now, is whether the outcome would be "forking" too. More important is, that for group 3 this would not solve anything in a helpful way: the columns (=6 elements) were, dependent on current group-3-presentation, either in "group IIIA/B" or "n/a" (=had no column number ever). I don't see any advantage to differentiate by writing "group 3, those formerly IIIA" and "group 3, those formery group n/a" (while still not identifying both new columns!).
- Adding to this: reusing any "A/B" notation would add a third notational form with A/B: which is excactly why that notation was abandoned in the first place, when being just two! And we should not forget that the A/B notation used Roman numbers ("IIIA"), never arabic ("3A").
- For these reasons, we should reject the suggestion in the bud.
- Still, to describe the issue Double sharp points to (re groups IIA, IIB), the old notation is perfectly fine & useful as is.
- Use sub-identifier. As presented in the Sandbh PT, both columns have a perfect identifier (descriptor) between the two:
- group 3(f-block), group 3(d-block)
- group 3f-block, group 3d-block
- 3 (f), 3 (d)
- 3f-block, 3d-block
- Exact form can be improved, point is that we use their block letter to distinct.
- Interpunction may matter; block letter always upright not italic. IMO, writing "3d, 3f" would be too confusing for example re elconfig.
- Another notation could be:
- group 3La, Ac; group 3Sc, Y, Lu, Lr.
- This is more descriptive, eg in introductionary texts.
- As before: by top element?. In general, we identified columns/groups by their top element: "The cobalt group" (which is not the same as "group VIII"). So in this case we could use "lanthium group", and "scandium group" respectively for the two columns? No, IMO. While probably correct by Red Book nomenclatura (cobalt & scandium are equal, right?), it may cause confusion at least. The cobalt example specifies one column out of three, and is never disputed. But the scandium example still is from an unstable PT form (not yet accepted scientifically; only when this bifurcation is accepted everywhere in RL stability might be in play). More fundamentally, as Sandbh described, the Sc, Y pair just as correct could be above La, Ac (as enwiki does today). For this, writing "scandium group" is ambivalent (ambiguous), and so requires clarification and includes implicit confusion. Now this could be true for the "group 3(?-block)" notation too (because a Sc, Y, La, Ac column is not a simple 3(?-block) column; but we know what we refer to because it is new notation. IOW, writing "scandium group" under a "group 3 = Sc, Y, La, Ac"-PT (=current enwiki) is a red flag.
- Elsewhere. Below, Sandbh mentions #Silberberg's table. in there, this notation would not fit because the block letters change within a column. That would require more descriptive text (at least once, then use like "3 (d/f), 3 (f only)"? Of course for ease of writing, we hope this form does not prevail ;-).
- My conclusion: When column distinction is needed, preferably the columns are identified by the block they are in. The notation should not be confusing (e.g., with electron configuration). For example:
- group 3f-block, group 3d-block
- -DePiep (talk) 09:47, 11 April 2019 (UTC)
- Far out! That's a considered post, DePiep.
- My simple view of group 3 as per the Silberberg table is that it's made up of Sc, Y, La, Lu, Ac, and Lr. It's more complicated that, of course, since there are two branches after Y. So, were you to write about group 3 from this perspective you'd have do a bit of comparative chemistry between Sc-Y-La-Ac, and Sc-Y-Lu-Lr, in order to be clear as to what's going on. How would you refer to each of this options/columns? Good question. There's only one group 3. I'd be inclined to call them the La branch, and the Lu branch. That would be analogous to the approach taken by Jensen (2018, p. 260) re groups 2 and 12: "This is resolved by placing the Ca branch in the (2+6) [i.e. group 2] and the Zn branch in [the same] group."
- Re IIA and IIB and a bifurcation after Mg, this doesn't have enough puff. Sidgwick, in his classic book, "The chemical elements and their compounds" (1950, vol. 1, p. 193), wrote "The difference in properties between the two subgroups is less in Group II than in Group I, but it is still very marked." [underline added] Sanderson (1967, p. 403) continues the theme: "Consisting of more compact, smaller, more electronegative atoms than those of Group [2], the [Group 12] elements have a chemistry that, although formally similar, is actually quite different." [underline added]
- On group 3, Sidgwick treats this as Sc, Y, La and the 14 succeeding elements up to Lu, and Ac. Sanderson does not have anything useful to say about group 3.
- Jensen WB 2018, "Richard Abegg and the periodic table", in Scerri E and Restrepo G (eds), Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table, Oxford University Press, New York, pp. 245−265
- Sanderson RT 1967, Inorganic chemistry, Reinhold Publishing Corporation, New York
- Sidgwick NV 1950, The chemical elements and their compounds, vol. 1, Clarendon Press, London
- Certainly the differences between {Ca, Sr, Ba, (Ra)} on one hand and {Zn, Cd, Hg} on the other are still very marked. But with their small size and higher electronegativity, it is nonetheless true that whenever Be and Mg behave differently from {Ca, Sr, Ba, (Ra)}, they pattern with the Zn group instead. A comparison table based on Jensen's is at group 12 element. I do agree that this is small potatoes compared to the difficulties we encounter at group 3, because while Be is somewhat equivocal, Mg is pretty resolute about siding with Ca; contrast that to Sc as a mini-Lu and Y hiding among the heavy lanthanides without anyone noticing. So I would just like to have the A and B labels back for a taste of the short table – of course, once we agree on which are the A groups and which are the B groups. (We can then call the branches of group 3 "IIIB(f)" and "IIIB(d)", or replacing B with A to taste.) Part of Jensen's arguments for why to consider group II as bifurcated is because he considers the Zn group exclusively a main group, but I think this is a little misguided because it gets into difficulties with Ag and Cn.
- I do think it is worth thinking a little about the special positions of Be and Al; they have the typical noble gas core of pre-transition metals, but are taking on a great deal of positive charge for their size in a manner more reminiscent of post-transition metals. But that's another story. Double sharp (talk) 20:38, 21 April 2019 (UTC)
Continuing the theme: Groups 2, 12
@Double sharp: A long time ago I saw Denker's Cylinder with bulges periodic table, and didn't think very much at all about it.
His long and rambling explanation didn't help, and still doesn't.
That said, I've struggled through it again and it does now appear to be quite clever.
As well as a bifurcated group 2, per Jensen, it has a trifurcated group 3, thus:
Group 2 = Be-Mg-
branch 1......-Ca-Sr-Ba-Ra (conventional group 2)
branch 2......-Zn-Cd-Hg-Cn (conventional group 12)
Group 3 = B-Al-
branch 1......-Sc-Y-
-La-Ac (conventional group 3)
-Lu-Lr
branch 2......-Ga-In-Tl-Nh (conventional group 13)
Group numbering in a 32-column table would then go like this:
1 2 3 Ce-Yb 3 4 5 6 7 8 9 10 11 2 3 14 15 16 17 18
Th-No
Riffing on Denker, the the first group 2 branch is "light", and the second one is "heavy"; and the three group 3 branches are light, medium, and heavy, respectively.
I'll spend some more time reading his page to see if can fully get my head around it. Sandbh (talk) 00:47, 27 April 2019 (UTC)
Isotopes of elements: the big table
I am working on the Big Table of Isotopes (like Isotopes of boron#List of isotopes). For now, I am standardising the header structure (peek preview: /testcases, /testcases2). Here are some questions for the WP:ELEMENTS community.
- The nucleonica reference: decay modes are referenced generically to nucleonica.net: "Universal Nuclide Chart". nucleonica.. The ref may be in the table header, or in a section #General references. That link is broken (irrespective of the registration wall). The homepage nucleonica.com (improved url into com) is working, but it still requires subscription. My question is: can I remove this reference from all the isotopes pages?
- Since it is a generic reference in the header or in the page, no specific data is sourced through it. Also, because of the registration wall I cannot check data. Also, we have similar generic sources like NUBASE2016, CRC, NNDC NuDat 2.x database available. Remove? -DePiep (talk) 13:13, 16 June 2019 (UTC)
- I'd say remove it. NUBASE2016 is more accessible and probably more recent, and is the main source I have been using to update various pages, including (especially) big isotope tables. ComplexRational (talk) 13:34, 16 June 2019 (UTC)
- OK I can safely remove those nucleonica links. -DePiep (talk) 21:32, 19 June 2019 (UTC)
 Done -DePiep (talk) 22:14, 21 June 2019 (UTC)
Done -DePiep (talk) 22:14, 21 June 2019 (UTC)
- About Article layout.
- I'll put the "List of isotopes" below/above* in the content consistently (an editorial choice, just to be consistent).
- I'll check all pages to conform MOS:LAYOUT. Per detail MOS:NOTES, I'll make sure below the content the references are ordered like this:
- Explanatory footnotes that give information which is too detailed or awkward to be in the body of the article
- Citation footnotes (either short citations or full citations) that connect specific material in the article with specific sources
- Full citations to sources, if short citations are used in the footnotes
- General references (full bibliographic citations to sources that were consulted in writing the article but that are not explicitly connected to any specific material in the article)
- We already know the isotopes pages often do have 'General references' (like NUBASE). I propose these four will be same level 2 (==) headers, but you might have a better idea. -DePiep (talk) 21:49, 19 June 2019 (UTC)
- *Polling/asking: should the Big Table be in top or below? -DePiep (talk) 00:36, 20 June 2019 (UTC)
- Above - although it is below in most articles, placing it above provides a tabulated summary before delving into specifics for each isotope. It also leads to a more consistent structure overall, as all 118 pages would begin with a lead section and table, and only then diverge when specific isotopes have sections. I'm open to other possibilities, though. ComplexRational (talk) 21:50, 21 June 2019 (UTC)
- OK. -DePiep (talk) 22:14, 21 June 2019 (UTC)
 Done. -DePiep (talk) 22:12, 27 June 2019 (UTC)
Done. -DePiep (talk) 22:12, 27 June 2019 (UTC)
- OK. -DePiep (talk) 22:14, 21 June 2019 (UTC)
- Above - although it is below in most articles, placing it above provides a tabulated summary before delving into specifics for each isotope. It also leads to a more consistent structure overall, as all 118 pages would begin with a lead section and table, and only then diverge when specific isotopes have sections. I'm open to other possibilities, though. ComplexRational (talk) 21:50, 21 June 2019 (UTC)
- *Polling/asking: should the Big Table be in top or below? -DePiep (talk) 00:36, 20 June 2019 (UTC)
- Not a 'stand alone' table - just noting
- As the table acts today, it is not a true stand alone (one cannot take the table apart without making info&ref&context incomplete). In short: if we want it to be self-contained, it should have a title, plus links to the generic references like NUBASE and AME2016, and maybe more. For now, I don't see the need – but this is how to think about things. -DePiep (talk) 00:58, 20 June 2019 (UTC)
The references
I see some issues with the generic references for the Big Table. In all articles, they are added as General reference (i.e., not inline appearing as [1]), like (Ds):
* Isotope masses from:
- {{AME2012}}
- {{NUBASE 2003}}
- Isotopic compositions and standard atomic masses from:
- {{CAWIA 2003}}
- {{CIAAW 2005}}
- Half-life, spin, and isomer data selected from the following sources.
- {{NUBASE 2003}}
- {{NNDC}}
- {{CRC85}}
Earlier data was from these sources of course. However, nowadays we would use {{NUBASE2016}} and {{NUBASE 2016 II}} (aka AME2016), and some data indeed has been checked and updated. The question is: how to do this sourcing, especially after updates? Should we make them inline refs (correctly reffed from Table header)? When do we add "2016" sources, when do we remove "2003" sources? (ping User:ComplexRational). -DePiep (talk) 08:34, 22 June 2019 (UTC)
- Or put a {{update}} in top of the List section, to be removed only when all outdated sources can be correctly removed? -DePiep (talk) 09:05, 22 June 2019 (UTC)
- @DePiep: The only way to accurately update everything requires a manual check of all 118 pages. I've only gotten the first 14 (Si) so far, so it may be necessary to tag the rest with {{update}} until I (or someone else) update them with 2016 data. As it stands, only the first 14 are sourced entirely to AME2016. In any case, though, the ref should only appear in the table header (or various places in the prose, but that must be done case-by-case). ComplexRational (talk) 11:42, 22 June 2019 (UTC)
- Sidenote: I have changed the two source templates: {{NUBASE 2016}} → {{NUBASE2016}}; and {{NUBASE 2016 II}} → {{AME2016 II}}. New names are as used in their title; also added param #1 options
|1=ref=,|1=name=so one can use same template in various situations (e.g. [3]) -- see template documentation. Only for those 2016 ones. -DePiep (talk) 15:50, 27 June 2019 (UTC)
- Sidenote: I have changed the two source templates: {{NUBASE 2016}} → {{NUBASE2016}}; and {{NUBASE 2016 II}} → {{AME2016 II}}. New names are as used in their title; also added param #1 options
- @DePiep: The only way to accurately update everything requires a manual check of all 118 pages. I've only gotten the first 14 (Si) so far, so it may be necessary to tag the rest with {{update}} until I (or someone else) update them with 2016 data. As it stands, only the first 14 are sourced entirely to AME2016. In any case, though, the ref should only appear in the table header (or various places in the prose, but that must be done case-by-case). ComplexRational (talk) 11:42, 22 June 2019 (UTC)
- Asking ComplexRational: when updating the isotopes big table (like in H), you added the reference {{NUBASE2016}} to column header "Decay modes". If I am correct, also the half-lives and other data is read from NUBASE. When templating the header with this reference, do I put it in that columnheader only, or is there a more precise solution? (We could repeat it in multiple columns, or add a description with the reference, as a prefix text). -DePiep (talk) 17:36, 27 June 2019 (UTC)
- @DePiep: I never considered it earlier, but it is more correct and precise to include {{NUBASE2016}} in the half-life, decay modes, nuclear spin, and representative isotopic composition columns, as all that data is taken from the same source. I'm not really sure how a prefix or single footnote for all four columns could work, and I would prefer to keep inline citations rather than have one at the bottom of the page not specifically referencing anything. ComplexRational (talk) 17:48, 27 June 2019 (UTC)
- @ComplexRational:. The table has no header, where such an inline reference would be appropriate, once. But we can put the citation once in the first column like "Nuclide[1]". Also, in there the prefix text could look like this:
- @DePiep: I never considered it earlier, but it is more correct and precise to include {{NUBASE2016}} in the half-life, decay modes, nuclear spin, and representative isotopic composition columns, as all that data is taken from the same source. I'm not really sure how a prefix or single footnote for all four columns could work, and I would prefer to keep inline citations rather than have one at the bottom of the page not specifically referencing anything. ComplexRational (talk) 17:48, 27 June 2019 (UTC)
References
- ^ Half-life, decay modes, nuclear spin, and representative isotopic composition is sourced in:
Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- (BTW, the header template will have the option
|ref=NUBASE2016, AME2016etc. to add them per article).
-DePiep (talk) 18:13, 27 June 2019 (UTC)
- @DePiep: That citation looks fine to me for the big table. ComplexRational (talk) 18:51, 27 June 2019 (UTC)
- OK. -DePiep (talk) 20:57, 27 June 2019 (UTC)
- @DePiep: That citation looks fine to me for the big table. ComplexRational (talk) 18:51, 27 June 2019 (UTC)
- So now we are using {{NUBASE2016}} and {{AME2016 II}} to source the isotopes data. However, I just discovered that isotopos abundances is not reported in these sources. For this, I assume we should use Meija, Juris; Coplen, Tyler B.; et al. (2016). "Isotopic compositions of the elements 2013 (IUPAC Technical Report)" (PDF). Pure and Applied Chemistry. 88 (3). doi:10.1515/pac-2015-0503. as the most recent source. Am I right? @ComplexRational:-DePiep (talk) 14:31, 28 June 2019 (UTC)
- @DePiep: Isotope abundance is in fact present in {{NUBASE2016}}. It is labelled 'IS' for primordial isotopes in the same column as decay modes for everything else. ComplexRational (talk) 16:08, 28 June 2019 (UTC)
- I see. The paper says these IS's are sourced in "[2011Be83]", which stands for:
- Pure and Applied Chemistry, 83, 397 M. Berglund, M.E. Wieser. This is the "2009" report.
- Berglund, Michael; Wieser, Michael E. (2011). "Isotopic compositions of the elements 2009 (IUPAC Technical Report)" (PDF). Pure and Applied Chemistry. 83: 397–410. doi:10.1351/PAC-REP-10-06-02.
- However, a more recent report is {{CIAAW2016abundance}}:
- Meija, Juris; Coplen, Tyler B.; et al. (2016). "Isotopic compositions of the elements 2013 (IUPAC Technical Report)" (PDF). Pure and Applied Chemistry. 88 (3). doi:10.1515/pac-2015-0503.
- I think best sourcing would be: first, update per NUBASE2016, including those 2011 IS values, and add the NUMABSE2016 ref to the table. Next, one could update per {{CIAAW2016abundance}} (2016), and add this source to the table. -DePiep (talk) 10:55, 29 June 2019 (UTC)
- Yes, I'll take a look at this. ComplexRational (talk) 12:55, 30 June 2019 (UTC)
- @DePiep: Isotope abundance is in fact present in {{NUBASE2016}}. It is labelled 'IS' for primordial isotopes in the same column as decay modes for everything else. ComplexRational (talk) 16:08, 28 June 2019 (UTC)
Element specifics - elements with traces only
@DePiep: You may want to change the list of elements to show/hide the natural occurrence column, as we are including traces of Tc, Pm, and Np. As a result, only elements from Am onward would be listed as entirely synthetic and thus should omit those twocolumns. This discussion on trace abundance began at User talk:ComplexRational#Oxygen-20, and I already added the columns in isotopes of Np (as an example). ComplexRational (talk) 12:55, 30 June 2019 (UTC)
- Done for Tc, Pm, Np [4] (in header table, in development). Currently, these elements appear in traces only: Tc, Pm, Po, At, Rn, Fr, Ra, Ac, Np. When deploying the template header. I will discover such changes when deploying, so they will pop up. -DePiep (talk) 14:36, 30 June 2019 (UTC)
Header template available
- {{Isotopes table}} (talk) -- /testcases (items), /testcases2 (example elements)
{{Navbox element isotopes/line}} I have prepared a template for the header of the Big Table (118 isotope articles).
Aim: replace all in-article wikicode table headers. Standardise the table re references, footnotes, and column structure. Creating a single point of editing. It is a technical change, while some textual improvements have been applied (see /testcases#Compare).
Supporting: allow for update into 2016 data (NUBASE2016 etc.). Option to add inline references for this (instead of current general references).
Not changed: the header does not change any row (individual isotope) in structure or data. However, empty columns might need removal of their pipe | (two rightmost columns).
- I will check this when we roll out the header in affected articles. ComplexRational (talk) 18:05, 6 July 2019 (UTC)
- We all will do that: a header changing editor better look around. -DePiep (talk) 22:21, 6 July 2019 (UTC)
Parameter options: {{Isotopes table}} allows adding some standard references (like {{NUBASE2016}}) and footnotes (like "nearly stable half-life"). Some columns are optional.
Future development: Table improvements can be considered, e.g. text changes, prevent double use of ()-marking, reorder columns. We could also consider making the whole table (all rows) templatised instead of hardcoded table | rows. This could be done in consideration with the 2016 update (helping).
- Perhaps in a similar manner to {{Infobox element isotopes}} and {{Isotopes summary}}? ComplexRational (talk) 18:05, 6 July 2019 (UTC)
- Yes! But to keep things simpified, this one step first (wow, these NUBASE details like # and () are a headache to footnote; they have double meanigs I discovered). -DePiep (talk) 22:21, 6 July 2019 (UTC)
Deployment: using the template is not a blind process. It requires attention to add the right options and check/remove notes present. We can start rolling out in a few articles and see if issues arise.
Proposal: pls check the documentation and testcases, and cast approval. (@ComplexRational:) -DePiep (talk) 10:02, 6 July 2019 (UTC)
- @DePiep: So far, the documentation and testcases look good. I made a few minor fixes; in particular, Tc and Pm appear to correctly be omitted from the column opt-out list, but it wasn't in the documentation. I would also like to suggest a central ver
headtical alignment for the header rows if possible, as the extra space looks somewhat awkward. Once this is addressed, we can start testing the header in articles. ComplexRational (talk) 18:05, 6 July 2019 (UTC)- You mean text spacing & positioning in the column headers? I have improved #compare. Could you explain? -DePiep (talk) 22:43, 6 July 2019 (UTC)
- Exactly that. I prefer the text of the new header, though IMO it looks better when positioned in the middle of the cells. (And I apologize for the typo which I now struck, as that may have been a source of confusion.) ComplexRational (talk) 00:00, 7 July 2019 (UTC)
- I'm not that enthousiastic about this (column header texts verticalwise in the middle not in top). As the old formatting shows, it creates an irregular top text line. Straight in top supports showing the table structure, which helps easier reading (we are unaware, but do experience this by the eye). Even more important with data rows below, which can add quite some chaotic looking texts & numbers. So all visible structure is welcome, while the alternative is esthetical only (if I understand you well).
- I will try this though: the footnotes[n 1] in the header can go to the bottom of the column header cell. -DePiep (talk) 08:43, 7 July 2019 (UTC) anbandoned (DePiep 20:57, 7 July 2019 (UTC))
- @ComplexRational: As I wrote, I do not support your idea re this header formatting. However, I do think this should pause or uphold the template rollout. The table header formatting can be adjusted later easily. Sure, we will contact when problems occur in the rollout. Point is, that the table setup is OK. I think it is. I propose we roll out this header thing, carefully (any ideas about orders/priorities?). -DePiep (talk) 21:08, 7 July 2019 (UTC)
- That's fine, we could also gauge how everyone else feels about the header. Other than that, there aren't any issues that I see - so we can start testing. I don't really care which specific articles, but for a list including almost all possible combinations of columns and some testcases, maybe try H, Fe, Cs, Pb, At (but remember to remove one column's pipes), U, Hs. ComplexRational (talk) 21:22, 7 July 2019 (UTC)
- OK. -DePiep (talk) 22:16, 7 July 2019 (UTC)
- We could use good wikilinks for headers spin & parity, and natural occurrence. But Spin (physics) and Parity (physics) are too abstract for this I think. Use Natural abundance? (shouldn't the word 'abundance' be used in the header even?). -DePiep (talk) 09:39, 8 July 2019 (UTC)
- Yes, links in the remaining columns would be helpful. Spin (physics) looks like the best link for that (also as Nuclear spin) redirects there; Parity (physics)#Nuclei would be more appropriate ad it points to the specific, relevant section; Natural abundance also seems fine for a link. Maybe parenthesize abundance (it also could be left as is)? ComplexRational (talk) 11:01, 8 July 2019 (UTC)
- Three links added, no text changes done. I am looking into {{CIAAW2016abundance}} on how the abundance values (our 2 columns) are defined, named & formatted. Will be back on this. -DePiep (talk) 13:01, 8 July 2019 (UTC)
- Yes, links in the remaining columns would be helpful. Spin (physics) looks like the best link for that (also as Nuclear spin) redirects there; Parity (physics)#Nuclei would be more appropriate ad it points to the specific, relevant section; Natural abundance also seems fine for a link. Maybe parenthesize abundance (it also could be left as is)? ComplexRational (talk) 11:01, 8 July 2019 (UTC)
- We could use good wikilinks for headers spin & parity, and natural occurrence. But Spin (physics) and Parity (physics) are too abstract for this I think. Use Natural abundance? (shouldn't the word 'abundance' be used in the header even?). -DePiep (talk) 09:39, 8 July 2019 (UTC)
- OK. -DePiep (talk) 22:16, 7 July 2019 (UTC)
- That's fine, we could also gauge how everyone else feels about the header. Other than that, there aren't any issues that I see - so we can start testing. I don't really care which specific articles, but for a list including almost all possible combinations of columns and some testcases, maybe try H, Fe, Cs, Pb, At (but remember to remove one column's pipes), U, Hs. ComplexRational (talk) 21:22, 7 July 2019 (UTC)
- @ComplexRational: As I wrote, I do not support your idea re this header formatting. However, I do think this should pause or uphold the template rollout. The table header formatting can be adjusted later easily. Sure, we will contact when problems occur in the rollout. Point is, that the table setup is OK. I think it is. I propose we roll out this header thing, carefully (any ideas about orders/priorities?). -DePiep (talk) 21:08, 7 July 2019 (UTC)
- Exactly that. I prefer the text of the new header, though IMO it looks better when positioned in the middle of the cells. (And I apologize for the typo which I now struck, as that may have been a source of confusion.) ComplexRational (talk) 00:00, 7 July 2019 (UTC)
- You mean text spacing & positioning in the column headers? I have improved #compare. Could you explain? -DePiep (talk) 22:43, 6 July 2019 (UTC)
- Option
|notes=noteadds extra column "Note" to the right, any text can go there in-row. In demo. -DePiep (talk) 13:01, 8 July 2019 (UTC) - Consulting WP:ELEMENTS regulars R8R, Double sharp, Sandbh, YBG, UtopianPoyzin, and ComplexRational:
- We are about to deploy the standardized header template for the big isotopes tables (see #compare old/new, demo U). Now ComplexRational and I differ in opnion about the formatting: should headertexts like "Nuclide" be in top of the header cell, or vertically in the middle, as the old form has? Arguments are above; Pro Vertically middle: reduces empty whitespace area; pro In top: enhances table structure for readibility. -DePiep (talk) 09:58, 8 July 2019 (UTC)
- In top to support table structure visually. -DePiep (talk) 12:48, 8 July 2019 (UTC)
- +1. Top is more readable. Sandbh (talk) 02:48, 9 July 2019 (UTC)
- It certainly does look better organized. I will only note that the Hs header seems overstretched vertically.--R8R (talk) 19:23, 8 July 2019 (UTC)
- (Fixed Hs. In such a flexible wikitable, superfluous newlines are tricky business). -DePiep (talk) 19:59, 8 July 2019 (UTC)
- In top to support table structure visually. -DePiep (talk) 12:48, 8 July 2019 (UTC)
Future improvements
To consider
- Check number formatting [5].
- When {{val}} is used, columns like cnuclide, half-life, spin could be made sortable
- Markings like ( ) and # are used twice (have two meanings). Separating those ie make different.
- Add column "Notes", where footnotes can go, like 277Ds "Not directly synthesized, occurs in decay chain of 285Fl".
- subrow Excitation energy in separate column? Is not that related to the Z, N, mass values.
-DePiep (talk) 08:30, 7 July 2019 (UTC)
- Let's go to {{NUBASE2016}} and {{AME2016 II}}, download the ascii file, and create these data pages (either enwiki or WD). Automate it! -DePiep (talk) 21:18, 7 July 2019 (UTC)
- Improve targets (define, describe, source) TNN (nuclear physics), TMS (nuclear physics), now redirects. (Their sources: see {{Isotopes table/ref group}}).-DePiep (talk) 17:14, 14 July 2019 (UTC)
- {{Actinides vs fission products}} may be correct, but graphically unclear and outdated technics. (After all, it was 2013 last time I edited it ;-) — little did I know). -DePiep (talk) 18:31, 14 July 2019 (UTC)
- ~dozen elements do have a regular naural abundance, but the column "range of variation" is empty. (don't loose sleep). -DePiep (talk) 19:42, 14 July 2019 (UTC)
- @DePiep: This is pretty straightforward, actually. There should be 21 of these elements; they are mononuclidic elements, they have no variation since the single primordial isotope trivially has 100% abundance. I can add these to the opt-out list, but we'll need an AWB run (if possible) to delete the extra column in the tables. These 21 elements are
Be, F, Na, Al, P, Sc, Mn, Co, As, Y, Nb, Rh, I, Cs, Pr, Tb, Ho, Tm, Au, Bi, Pa.
ComplexRational (talk) 21:30, 14 July 2019 (UTC)- Yes I understand, I thought of these being monoisotopic elements (though there are elements that have this point and are not monoisotopic). It is just: it showed up to me now that I edit the table headers, checking the result visually. I explicitly decided to mention & postpone this issue ("possibly missing data"), because the table header change is complicated enough. I first want to have these 118 table headers right. -DePiep (talk) 21:37, 14 July 2019 (UTC)
- @ComplexRational: BTW, do not change any opt-out list for now. a. I'm editing in this, and b. monoisotopic is different issue, not 'traces only'. -DePiep (talk) 21:41, 14 July 2019 (UTC)
- @DePiep: I haven't changed anything yet; I'm just leaving it here so that you can proceed when ready. (And BTW, mononuclidic and monoisotopic are not the same.) ComplexRational (talk) 21:48, 14 July 2019 (UTC)
- Very distracting. You missed my point that I encountered isotopes lists that *do not* have a natural variance range value (final column is empty), while these *do* have natural abundances that are =/= "1.000". If I am correct, *that* is an issue, not the mono-whatever wording. Anyway, postponed. -DePiep (talk) 21:55, 14 July 2019 (UTC)
- I'm not exactly sure what you mean. I just checked every one of those elements and the final column is indeed empty (or in the case of Y, not present at all), but they of course have representative isotopic compositions with the single isotope having 1.000 - we'll look at these details later. If I am still missing your point, please clarify. ComplexRational (talk) 22:07, 14 July 2019 (UTC)
- My point is: I noted today, while editing the Big Table header into {{Isotopes table}} & visually checking the resulting table (118×): some element isotopes have a "1.000" natural abundance, but do not have a value in their corresponding "range" column (next to it). Can happen. Could be monoisotopic elements, but maybe I get that definition wrong. (future Q: keep that empty column? Add some new footnote?). THEN I also saw elements with a non1.000 abundance, but still not having a range (empty column). See Ag. For those cases, I question: probably the recent source ({{CIAAW2016abundance}}) could have a value for that range.
- (Add to the confusion: Sb has values like 12.345(2) but not a range value).
- I have no links, because I do not want to spend (mind)time on this. The header change is very intensive.
- How to proceed? I will finish the standard header first. Secondary lookes & edits are required. Then some look into this would be good (appearance, footnotes, stray footnotes). Data update into "2016" can continue always. Maybe even change the new header. -DePiep (talk) 23:48, 14 July 2019 (UTC)
- I'm not exactly sure what you mean. I just checked every one of those elements and the final column is indeed empty (or in the case of Y, not present at all), but they of course have representative isotopic compositions with the single isotope having 1.000 - we'll look at these details later. If I am still missing your point, please clarify. ComplexRational (talk) 22:07, 14 July 2019 (UTC)
- Very distracting. You missed my point that I encountered isotopes lists that *do not* have a natural variance range value (final column is empty), while these *do* have natural abundances that are =/= "1.000". If I am correct, *that* is an issue, not the mono-whatever wording. Anyway, postponed. -DePiep (talk) 21:55, 14 July 2019 (UTC)
- @DePiep: I haven't changed anything yet; I'm just leaving it here so that you can proceed when ready. (And BTW, mononuclidic and monoisotopic are not the same.) ComplexRational (talk) 21:48, 14 July 2019 (UTC)
- @ComplexRational: BTW, do not change any opt-out list for now. a. I'm editing in this, and b. monoisotopic is different issue, not 'traces only'. -DePiep (talk) 21:41, 14 July 2019 (UTC)
- Yes I understand, I thought of these being monoisotopic elements (though there are elements that have this point and are not monoisotopic). It is just: it showed up to me now that I edit the table headers, checking the result visually. I explicitly decided to mention & postpone this issue ("possibly missing data"), because the table header change is complicated enough. I first want to have these 118 table headers right. -DePiep (talk) 21:37, 14 July 2019 (UTC)
- @DePiep: This is pretty straightforward, actually. There should be 21 of these elements; they are mononuclidic elements, they have no variation since the single primordial isotope trivially has 100% abundance. I can add these to the opt-out list, but we'll need an AWB run (if possible) to delete the extra column in the tables. These 21 elements are
Natural occurence and variation (abundances)
The table has two columns re natural occurrence and variation therein. I have looked into their origin: which data is in there, how is it defined & named.
Source appears to be {{CIAAW2016abundance}}[1] ([6]) or its predecessors. Columns are described/defined on p. 295 (=pdf p.3/14; column 9 only so in the column 6 paragraph). Below is a table with corresponding column headers (enwiki – CIAAW), and example 50V.
| Nuclide |
Z | N | Isotopic mass (Da) |
Half-life |
Decay mode |
Daughter isotope |
Spin and parity |
Natural abundance (mole fraction) | Note | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal proportion | Range of variation | ||||||||||||||||||
| 50V[n 1] | 23 | 27 | 49.9471585(11) | 1.4(4)×1017 y | EC (83%) | 50Ti | 6+ | 0.00250(4) | 0.002487–0.002502 | From V isotopes, as of 8 July 2019[update] | |||||||||
| β− (17%) | 50Cr | ||||||||||||||||||
| CIAAW table[7] | col 9 | col 4 | col 6 (not used in enwiki table) | ||||||||||||||||
| Representative isotopic abundance (isotope-amount fraction, x) |
Observed interval of isotope-abundance variation in natural materials (isotope-amount fraction, x) |
Best measurement of isotopic abundances from a single terrestrial source (isotope-amount fraction, x) | |||||||||||||||||
| 50V | 0.002 50(10) | [0.002 487, 0.002 502] | 0.002 497(6) | ||||||||||||||||
| YBG, idea 1 | ... earlier headers not represented here ... | Isotopic composition (mole fraction) | Note | ||||||||||||||||
| Representative value | Range of variation | ||||||||||||||||||
| 50V | 23 | 27 | 49.9471585(11) | 1.4(4)×1017 y | EC (83%) | 50Ti | 6+ | 0.00250(4) | 0.002487–0.002502 | See post by YBG below. | |||||||||
| β− (17%) | 50Cr | ||||||||||||||||||
| idea 1b [DePiep] | ... earlier headers not represented here ... | Natural abundance (mole fraction) | Note | ||||||||||||||||
| Isotopic abundance | Natural variation | ||||||||||||||||||
| 50V | 23 | 27 | 49.9471585(11) | 1.4(4)×1017 y | EC (83%) | 50Ti | 6+ | 0.00250(4) | 0.002487–0.002502 | Text changes; taken from IUPAC above | |||||||||
| β− (17%) | 50Cr
| ||||||||||||||||||
| idea 1c [YBG] | ... earlier headers not represented here ... | Spin & parity |
Isotope abundance (mole fraction) | Note | |||||||||||||||
| Normal proportion | Range of variation | ||||||||||||||||||
| 50V | 23 | 27 | 49.9471585(11) | 1.4(4)×1017 y | EC (83%) | 50Ti | 6+ | 0.00250(4) | 0.002487–0.002502 | per discussion below 15:33, 9 July 2019 (UTC) | |||||||||
| β− (17%) | 50Cr | ||||||||||||||||||
| This table header & footer: | |||||||||||||||||||
References
- Remarks & conclusions
1. The two enwiki column headers are not very helpful (where from? old definitions?). Working in WP:ELEMENTS, I am very used to "abundance" and "natural occurrance" (e.g., in atomic weight definitions). I propose to change column titles. For example, from CIAAW:
- "Isotopic abundance" and
- "Abundance variation in natural materials" (or "Variation in natural abundance"?)
- And quanity "(isotope-amount fraction)"
- More concise texts would be welcome. Can we omit the "x"? Link to Natural abundance.
2. The range is an interval. Formal notation is [a, b] not a–b. It is not an uncertainty, but an observed variation. (todo: adjust |notes=unc[] both text & code). In the future, we might change the value formatting.
3. "Column 6" has a most precise measurement, we do not use this value. Any need to add this? (no opinion from me).
4. The data can use an Update 2016.
5. We can spread the standard table now, and change these things afterwards.
Comments? -DePiep (talk) 14:05, 8 July 2019 (UTC)
YBG's idea
- (post by YBG moved here into dedicated subsection -DePiep (talk) 10:03, 9 July 2019 (UTC)):
- The two isotopic composition columns look rather ugly because (a) they have so many linewraps and (b) there is a lot of common text between the two. Seems we could factor out the common text into a separate line and make things much neater and more understandable. I see from the test cases that this will be complicated by the fact that both columns don't always appear, but I'd still say it is well worth the effort. YBG (talk) 04:48, 9 July 2019 (UTC)
- Here's what I have in mind:
| current |
| ||||||||||
| idea 1 |
| ||||||||||
| idea 2 |
| ||||||||||
YBG (talk) 04:48, 9 July 2019 (UTC)
- Like it, esp idea 1. I suggest text changes (see demo table), mainly to follow IUPAC column definitions and because to me they are more meaningful. -DePiep (talk) 10:53, 9 July 2019 (UTC)
- Agree with DePiep, idea 1 is a much cleaner representation of the concept and sticks to the most precise terminology. 1a and 1b are equally okay to me, but I support consistency with IUPAC in principle. @DePiep: If this change is made, would you also consider re-wrapping Nuclear spin and parity into three lines? With both adjustments, a lot of the blank space I mentioned earlier should be eliminated. ComplexRational (talk) 11:06, 9 July 2019 (UTC)
- My text proposals are a bit rough, maybe a finer wording is possible. re spin: yes, will do that; btw, do we need keep the word "Nuclear" in there? Looks like all the context is 'nuclear'. -DePiep (talk) 11:17, 9 July 2019 (UTC)
- I see nothing in the wording that really needs to be changed; maybe nuclear is a redundant definition but I feel it provides appropriate context (e.g. not to be confused with even and odd nuclei). My suggestion was more along the lines of nuclear<br/>spin and<br/>parity; that reduces the text to three lines with a minimal increase in column width. ComplexRational (talk) 14:36, 9 July 2019 (UTC)
- DePiep Thank you for moving my comment to the appropriate location; I sometimes get a bit testy about refactoring talk, but this one was absolutely spot on.
- ComplexRational What about spin &<br/>parity? The hard break might not even be necessary - the software might do the right thing automagically.
- I've added a new idea. I like the term "fraction" or something like that - something to emphasize that the values are a proportion of this isotope relative to 1.00 for all isotopes of the same element, which is not exactly what one would expect from the term "natural abundance". Also, I note that idea 1b uses the terms "abundance" and "natural" twice each, which seems a bit odd. In addition to "Isotopic abundance // Normal proportion / Range of variation" shown above, another idea would be "Natural abundance // Isotopic abundance / Natural variation".
- YBG (talk) 15:33, 9 July 2019 (UTC)
- re automatic linebreak, not using <br/>: usually fine, but in this wikitable it is troublesome to get that action right (optional columns & rows also require linebreaks, lots of linebreak interactions then; and <div>s I could not get to work).
- re wording: sure my initial text proposals are clumsy, but I definitely want to use the IUPAC words, the old words look unclear to me (and nobody here has clarified or ecxplained their background. I guess that they are old-timers & obsolete). So I can support the latest trio YBG mentions (idea 1c; but write "Isotopic"?). Wikilink ideas welcome. I will build the new structure shortly. -DePiep (talk) 16:31, 9 July 2019 (UTC)
- Detail: 10 elements appear in traces only (eg Po), so only the lefthand column is in there, mentioning "Traces" for some isotopes (no fraction numnbers). Headertext then be simple "Isotopic/e abundance"? -DePiep (talk) 16:43, 9 July 2019 (UTC)
 Done along line of YBG. Whitespace & newlines looks nicely reduced. Structure variants should be working OK, texts can always be tweaked later on. See /testcases (items), /testcases2 (example elements). Any blocking issues? -DePiep (talk) 17:22, 10 July 2019 (UTC)
Done along line of YBG. Whitespace & newlines looks nicely reduced. Structure variants should be working OK, texts can always be tweaked later on. See /testcases (items), /testcases2 (example elements). Any blocking issues? -DePiep (talk) 17:22, 10 July 2019 (UTC)
- @DePiep: Looks good. I see no issues in article testing/implementation. ComplexRational (talk) 20:04, 10 July 2019 (UTC)
- Thanks, appreciated. We take a sleep, then look at the tests again, and then slowly roll it out. I'll notify & ping this thread. -DePiep (talk) 20:26, 10 July 2019 (UTC)
- @DePiep: Looks good. I see no issues in article testing/implementation. ComplexRational (talk) 20:04, 10 July 2019 (UTC)
- I see nothing in the wording that really needs to be changed; maybe nuclear is a redundant definition but I feel it provides appropriate context (e.g. not to be confused with even and odd nuclei). My suggestion was more along the lines of nuclear<br/>spin and<br/>parity; that reduces the text to three lines with a minimal increase in column width. ComplexRational (talk) 14:36, 9 July 2019 (UTC)
- My text proposals are a bit rough, maybe a finer wording is possible. re spin: yes, will do that; btw, do we need keep the word "Nuclear" in there? Looks like all the context is 'nuclear'. -DePiep (talk) 11:17, 9 July 2019 (UTC)
- Agree with DePiep, idea 1 is a much cleaner representation of the concept and sticks to the most precise terminology. 1a and 1b are equally okay to me, but I support consistency with IUPAC in principle. @DePiep: If this change is made, would you also consider re-wrapping Nuclear spin and parity into three lines? With both adjustments, a lot of the blank space I mentioned earlier should be eliminated. ComplexRational (talk) 11:06, 9 July 2019 (UTC)
- Like it, esp idea 1. I suggest text changes (see demo table), mainly to follow IUPAC column definitions and because to me they are more meaningful. -DePiep (talk) 10:53, 9 July 2019 (UTC)
Deploying
I have started deploying {{Isotopes table}}. H, C. Todo, by check:
- Replace header with the template
- Replace footer with footer template
- Add when
|refs=NUBASE2016, AME2016 II, CIAAW2016are used - Remove outdated general references (NUBASE2003 etc).
- Remove unnecessary table notes
- Reposition stray generic "*" bullet table notes
- Check which
|notes=are required - Not doing: update data into NUBASE2016, AME2016 II
- Some checks may need a second run. -DePiep (talk) 21:39, 13 July 2019 (UTC)
- Created
new Redirects: TNN (nuclear physics), TMS (nuclear physics). Used in {{Isotopes table/ref group}} (their defining refs are in there), heavily mentioned in sources {{NUBASE2016}} and {{AME2016 II}}, and so the Isotopes of ... articles. They could have better target links (with abbr defined + described + sourced in an article). -DePiep (talk) 17:14, 14 July 2019 (UTC)
- Done are (rought list)
- Batch 1: having AME2016 ref. -H- 8x. Getting the touch of it.
- Batch 2: SHE's, syn only, no natural abu, nu NUBAS2016 now, -Og- 7x
- Batch 3: alle non-naturals or trace only, usually no NUBASE2016, (Po issues solved) -Cn- ~30x.
- Batch 4: specials like Th, -U- (2x) -DePiep (talk) 18:44, 14 July 2019 (UTC)
- Batch 5: all those *not* having update NUBASE2016. -DePiep (talk) 23:51, 14 July 2019 (UTC)
- Batch 6: those with NUBASE2016 and AME 2916.
 Done. No broken pages left behind AFAIK. Re the ~12+
Done. No broken pages left behind AFAIK. Re the ~12+ |ref=and|notes=options. to be checked. (Decay mode abbr's must be added anyway). -DePiep (talk) 01:02, 15 July 2019 (UTC)
Deploying done - report
The tableheader now is deployed in all Isotopes articles. Some notes:
- Notes
- FYI: many footnotes appear in Isotopes of indium.
- Update into "NUBASE2016" can proceed as before. Documentation shows that references
|refs=NUBASE2016, AME2016 II, CIAAW2016abundancecan be added automatically (and no others, at the moment). - Different notation in abundances: some isotopes have abundance fraction "1.000", with no range variation (Isotopes of niobium). Some note to be made in that range column? Or remove that (empty) column?
- Other isotope abundances are noted as (Isotopes of antimony): "0.5721(5)" and "0.4279(5)". Unclear is whether this is describing the "range" we regularly note (per source); a probability range is noted as [0.999816, 0.999974] in the source, and 0.999816–0.999974 here (ie incorrect punctuation). The ( ) notation is about 1 σ, a range says something different. I do not know if we should make adjustments for this ( ) probability notation (in header/footnote text?). H even has both in one row.
- If no issues are present, I plan to close & archive this thread. New issues can be raised in a new WT:ELEMENTS thread.
History of aluminium TFA nearing
On June 7, history of aluminium will run as WP:Today's featured article. While reading the blurb, I noticed how unreflective it was of the 20th (and, by extension, 21st) century and then I noticed the same was true of the article's lead section. I tried to fix the article itself but I think I won't have the opportunity to fix the blurb as well. Could someone please update the blurb so that the century when aluminum became famous to the general public is reflected as well? The information in the lead section of the article should suffice. You only need to know the blurb should be ~975 characters long.--R8R (talk) 23:07, 2 June 2019 (UTC)
- @R8R: I made some changes to the blurb; it is now 1036 characters long and includes a brief summary of aluminium's applications and importance during the 20th century. Could this work? ComplexRational (talk) 03:12, 3 June 2019 (UTC)
- I originally wanted to leave it as it is due to lack of ideas and time to invest in finding a solution but in the back of my mind, I have devised an idea overnight. Will try to implement it tonight.
- Also, 1036 does seem like too many. The supposed length for a TFA blurb used to be 1000 characters originally and only comparably recently was it lowered to 975---see, they do think there's a difference even between those two. I think I'll fit into that requirement more or less smoothly, though.--R8R (talk) 11:53, 3 June 2019 (UTC)
- I've made some changes. I think the text is better now because it more closely focuses on the metal itself rather than various names surrounding it, which seems appropriate given the room limitations and the article's title.--R8R (talk) 22:28, 3 June 2019 (UTC)
- I've wordsmithed it down below 800 chars without (I believe) removing any content. YBG (talk) 04:43, 4 June 2019 (UTC)
- I've made some changes. I think the text is better now because it more closely focuses on the metal itself rather than various names surrounding it, which seems appropriate given the room limitations and the article's title.--R8R (talk) 22:28, 3 June 2019 (UTC)
Feynmanium Element 137 listed at Redirects for discussion

An editor has asked for a discussion to address the redirect Feynmanium Element 137. Please participate in the redirect discussion if you wish to do so. ComplexRational (talk) 18:36, 22 July 2019 (UTC)
- this activates the AA bot. -DePiep (talk) 12:07, 23 July 2019 (UTC)
Alkyl compounds of superheavy elements
10.1524/ract.1973.19.2.69 (in German). Double sharp (talk) 04:36, 5 July 2019 (UTC)
Colors for element groups - any progress?
I remember earlier that I was reading about you guys deciding to recolor the Wikipedia periodic table groups. (I don't know why - in order to make it more accessible to the colorblind? To distinguish categories better? Something else?) I haven't heard of anything in a while or how it would proceed.
Personally, I'd probably make the metals all a shade of one color and the nonmetals a contrasting shade. I thought about this back in 2012 (!) and decided at the time to use a contrast of warm and cool colors, and make the metalloids an intermediate color between the two. I'm not sure how to format my suggestion, so here goes nothing:
Metals (warm colors, generally would appear as yellow to red-green colorblind individuals) Alkali = red Alkaline earth = red-orange Lanthanoids = light brown (technically the correct term according to the IUPAC) Actinoids = dark brown Transition metals = golden orange Post-transition metals = yellow
Metalloids = yellow-green
Nonmetals (cool colors, generally would appear as blue to red-green colorblind individuals)
"Other nonmetals" (the term used at the time) = green Halogens = blue Noble gases = violet (I'd probably do something different now that the halogens are not separate from the other guys anymore.)
What do you guys think? ― Дрейгорич / Dreigorich Talk 04:13, 5 July 2019 (UTC)
- Thanks. Changing of these colors (we call those sets "categories" at enwiki, no common name is available), is still on the agenda. However, the requirements we must fulfill are tough, and there are little degrees of freedom left. We must take care of: web contrast for readability, color blindness, usefulness as identifier (i.e., recognise from/towards the legend below), distinction between each other. All this for nine categories plus one 'unknown'.
- As for your line of thinking: Adding one more requirement like "warm" (or associative) colors is cultural, and therefor not recommended. Besides, they are very hard to reason & argue about. Also, given the requirements mentioned for the 9+1 colors, there are not much freedoms left (actually I think the freedoms are negative ;-) ).
- If there would be any choice left in the end (say, some colors could be swapped without issue), people here have opted for a nice, evenly spread of colors, tones, shades over the whole so that the periodic table, at a distance, looks nice for the eye. -DePiep (talk) 06:17, 5 July 2019 (UTC)
Interesting ref for superheavies
https://www.degruyter.com/view/j/pac.2018.90.issue-11/pac-2018-0918/pac-2018-0918.xml
- Let's date this post. Mayb a sign later on -DePiep (talk) 22:53, 7 July 2019 (UTC)
Is the kettle black?
Is this a good practice? Certainly DePiep’s frequent [undo] pushing irritates me, but—from my part—all these things are (yet) merely content disputes. I’m trying to push the pointless flamewar under {{hidden}}, but DePiep responds with cryptic accusations, some in the edit summary. May DePiep cease to call me a black kettle? Incnis Mrsi (talk) 15:56, 14 August 2019 (UTC)
- In this thread, your opening post contained aspersion re me. When I called you out on that, it is not up to you to hide a call for responsbility. BTW, let's see how your simultaneous endeavour with similar pattern ends. -DePiep (talk) 16:13, 15 August 2019 (UTC)
- Does DePiep expect assistance in edit-warring in Template:Periodic table (group 13) (edit | talk | history | links | watch | logs) and Group (periodic table) (edit | talk | history | links | watch | logs)? Let’s see… Incnis Mrsi (talk) 10:14, 17 August 2019 (UTC)
Infobox element, micro PT: trouble in paradise
Wikipedia provides an app that gives access to WP (next to, on mobile one can open an internet browser an access the enwiki website). All fine.
Now that app, on an Apple iphone I checked, the {{infobox element}} does not show the micro "<element> in the Periodic table" correctly — or not at all.
Noting for now. To reproduce this fail: in the WP app, check the Uranium infobox. -DePiep (talk) 20:44, 27 July 2019 (UTC)
- @DePiep: I just did a check in the WP app on both iOS and Android devices, and found that the micro PT in {{Infobox uranium}} renders correctly both in the article and template page, exactly as it does and should on desktop. I don't see the problem you describe. ComplexRational (talk) 15:55, 30 July 2019 (UTC)
- Good news, this says that I must check my own mobile settings etc. -DePiep (talk) 16:04, 30 July 2019 (UTC)
Two anachronistic proposals
What would our WP periodic table look like if it were organized after the fashion of Mendelev? There are two different (and equally interesting) ways to approach this
- If wikipedia had been around in his time, how would the proto-WP:ELEM have represented the then current state of scientific knowledge?
- What would a WP PT look like to represent the current state of scientific knowledge organized after the fashion of Mendelev?
Note that (1) would not show atomic numbers, but (2) would. (2) would definitely include our WP color categories, and I think (1) would be better with those colors even though it would be slightly anachronistic. Anyone want to have a go at building either of these? YBG (talk) 20:35, 27 July 2019 (UTC)
- Not sure what you think of, but I started {{Periodic table (Reihen and periods)}} (unpublished). It should bridge 1871–2019 (really, it this excercise taught me a lot). -DePiep (talk) 21:40, 27 July 2019 (UTC)
- Wouldn't (2) just be a short-form periodic table? Double sharp (talk) 09:58, 31 July 2019 (UTC)
