Thorium trichloride
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Trichlorothorium
| |
| Other names
Thorium(III) chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cl3Th | |
| Molar mass | 338.39 g·mol−1 |
| Appearance | crystals |
| Related compounds | |
Related compounds
|
Americium trichloride, uranium trichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thorium trichloride is a binary inorganic compound of thorium metal and chlorine with the chemical formula ThCl3.[1][2][3]
Synthesis
[edit]The compound can be prepared by reducing thorium tetrachloride at 800°C:[4]
- 3Th + ThCl4 → 4ThCl3
Also a reaction of both elements:[5]
- 2Th + 3Cl2 → 2ThCl3
Other reactions are also known.[6]
Physical properties
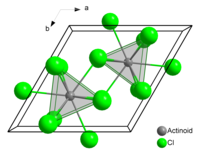
[edit]The compound forms crystals of the uranium trichloride crystal system.
Chemical properties
[edit]Above 630 °C thorium trichloride dissociates into the dichloride and tetrachloride.
Uses
[edit]Thorium trichloride is supposed[clarification needed] to be used in a dual fluid reactor as reactor fuel.[7]
References
[edit]- ^ Lau, K. H.; Hildenbrand, D. L. (1990). "High-temperature equilibrium studies of the gaseous thorium chlorides". J. Chem. Phys. 92 (10): 6124. Bibcode:1990JChPh..92.6124L. doi:10.1063/1.458335. Retrieved 5 April 2024.
- ^ Buschbeck, Karl-Christian (1985). Gmelin Handbook of Inorganic Chemistry. Springer-Verlag. p. 34. ISBN 978-3-540-93515-5. Retrieved 5 April 2024.
- ^ Bagnall, Kenneth W.; Kanellakopulos, Basil (21 December 1984). Coordination Compounds. Springer Berlin Heidelberg. p. 22. ISBN 978-3-540-93515-5. Retrieved 5 April 2024.
- ^ Annual Summary Research Report of Chemistry, Engineering, Metallurgy, Physics and Reactor Divisions. Ames Laboratoty. 1965. p. 1-9. Retrieved 5 April 2024.
- ^ Bulletin. U.S. Government Printing Office. 1962. p. 116. Retrieved 5 April 2024.
- ^ Reactor Fuel Processing. U.S. Argonne National Laboratory. 1961. p. 42. Retrieved 5 April 2024.
- ^ "The Dual Fluid Reactor" (PDF). Institute for Solid-State Nuclear Physics gGmbH. Retrieved 5 April 2024.
