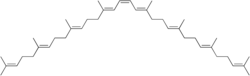
Phytoene
| |

| |
| Names | |
|---|---|
| IUPAC name
15-cis-7,8,11,12,7′,8′,11′,12′-Octahydro-ψ,ψ-carotene
| |
| Systematic IUPAC name
(6E,10E,14E,16Z,18E,22E,26E)-2,6,10,14,19,23,27,31-Octamethyldotriaconta-2,6,10,14,16,18,22,26,30-nonaene | |
| Other names
15-cis-Phytoene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C40H64 | |
| Molar mass | 544.952 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phytoene (/ˈfaɪtoʊiːn/) is a 40-carbon intermediate in the biosynthesis of carotenoids.[1] The synthesis of phytoene is the first committed step in the synthesis of carotenoids in plants. Phytoene is produced from two molecules of geranylgeranyl pyrophosphate (GGPP) by the action of the enzyme phytoene synthase.[2] The two GGPP molecules are condensed together followed by removal of diphosphate and proton shift leading to the formation of phytoene.
Dietary phytoene and phytofluene are found in a number of human tissues including the liver, lung, breast, prostate, colon, and skin.[3] Accumulation of these carotenoids in the skin may protect the skin by several mechanisms: acting as UV absorbers, as antioxidants, and as anti-inflammatory agents.[4][5]
Structure
[edit]Phytoene is a symmetric molecule containing three conjugated double bonds. Phytoene has a UV-Vis absorption spectrum typical for a triply conjugated system with its main absorption maximum in the UVB range at 286 nm and with ε1% of 915.[clarification needed][citation needed]
Sources
[edit]Analysis of several fruits and vegetables showed that phytoene and phytofluene are found in majority of fruits and vegetables.[6] In contrast to all other carotenoids, phytoene and phytofluene, the first carotenoid precursors in the biosynthetic pathway of other carotenoids, absorb light in the UV range.
History
[edit]The structure of phytoene was established and proven by total synthesis, by the Basil Weedon group in 1966.[7]
References
[edit]- ^ "Carotenoid Biosynthesis". Archived from the original on 2016-11-05. Retrieved 2009-02-25.
- ^ Phytoene synthase
- ^ Khachik, Frederick; Carvalho, Lorena; Bernstein, Paul S.; Muir, Garth J.; Zhao, Da-You; Katz, Nikita B. (November 2002). "Chemistry, Distribution, and Metabolism of Tomato Carotenoids and Their Impact on Human Health". Experimental Biology and Medicine. 227 (10): 845–851. doi:10.1177/153537020222701002. ISSN 1535-3702. PMID 12424324. S2CID 22671223.
- ^ Aust, W. Stahl, H. Sies, H. Tronnier, U. Heinrich (2005). "Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema". Int J Vitam Nutr Res. 75 (1): 54–60. doi:10.1024/0300-9831.75.1.54. PMID 15830922.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ B. B. Fuller; D. R. Smith; A. J. Howerton; D. Kern (2006). "Anti-inflammatory effects of CoQ10 and colorless carotenoids". Journal of Cosmetic Dermatology. 5 (1): 30–38. doi:10.1111/j.1473-2165.2006.00220.x. PMID 17173569. S2CID 9407768.
- ^ Khachik, F., G.R. Beecher, M.B. Goli, and W.R. Lusby (1991). "Separation, identifi cation, and quantification of carotenoids in fruits, vegetables and human plasma by high performance liquid chromatography". Pure Appl. Chem. 63 (1): 71–80. doi:10.1351/pac199163010071. S2CID 36564853.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. B. Davis; L. M. Jackman; P. T. Siddons; B. C. L. Weedon (1966). "Carotenoids and related compounds. XV. The structure and synthesis of phytoene, phytofluene, zeta-carotene, and neurosporene". J. Chem. Soc. C: 2154–2165. doi:10.1039/J39660002154.
Further reading
[edit]- Oppen-Bezalel, Liki von; Shaish, Aviv (2019-05-07) [2009]. "Application of the Colorless Carotenoids, Phytoene, and Phytofluene in Cosmetics, Wellness, Nutrition, and Therapeutics". The Alga Dunaliella. CRC Press. doi:10.1201/9780429061639-18. ISBN 978-0-429-06163-9. OCLC 1100588292.
