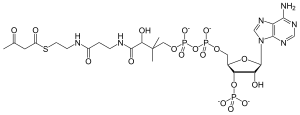
Acetoacetyl-CoA
| |
| Names | |
|---|---|
| IUPAC name
3′-O-Phosphonoadenosine 5′-[(3R)-3-hydroxy-2,2-dimethyl-4-oxo-4-{[3-oxo-3-({2-[(3-oxobutanoyl)sulfanyl]ethyl}amino)propyl]amino}butyl dihydrogen diphosphate]
| |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} O3-[(3R)-3-hydroxy-2,2-dimethyl-4-oxo-4-{[3-oxo-3-({2-[(3-oxobutanoyl)sulfanyl]ethyl}amino)propyl]amino}butyl] dihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.378 |
| MeSH | acetoacetyl+CoA |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H40N7O18P3S | |
| Molar mass | 851.61 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Acetoacetyl CoA is the precursor of HMG-CoA in the mevalonate pathway, which is essential for cholesterol biosynthesis. It also takes a similar role in the ketone bodies synthesis (ketogenesis) pathway of the liver.[1] In the ketone bodies digestion pathway (in the tissue), it is no longer associated with having HMG-CoA as a product or as a reactant.
It is created from acetyl-CoA, a thioester, which reacts with the enolate of a second molecule of acetyl-CoA in a Claisen condensation reaction,[2] and it is acted upon by HMG-CoA synthase to form HMG-CoA.[1] During the metabolism of leucine, this last reaction is reversed. Some individuals may experience Acetoacetyl-CoA deficiency.[3] This deficiency is classified as a disorder ketone body and isoleucine metabolism that can be inherited.[citation needed] Additional mutations include those with the enzymes within pathways related to Acetoacetyl CoA, including Beta-Ketothiolase deficiency and Mitochondrial 3-hydroxy-3-methylglutaryl-CoA Synthase mutation.

Additionally, it reacts with NADPH-dependent acetoacetyl-coenzyme A reductase, also known as PhaB, in a pathway that produces polyester polyhydroxyalkanoate (PHA). The reduction of acetoacetyl-coA by Pha creates (R)-3-hydroxybutyryl-CoA, which polymerizes to PHA.[4] The pathway is present in bacteria such as Ralstonia eutropha and the PCC6803 strain of Synechocystis.[5] Mover over, Acetoacetyl-CoA is involved with neuronal development involving lipogenesis and providing fats and cholesterol for neuronal cells.
Mutations
[edit]Mitochondrial acetoacetyl-CoA thiolase, also known as thiolase II, the enzyme responsible for catalyzing the synthesis of acetoacetyl-CoA within ketogenesis as mentioned, is also involved within acetoacetyl-CoA cleavage in ketolysis. It is observed to play a role within cleavage of acetyl-CoA from acetoacetyl-CoA and 2-methylacetoacetyl-CoA. The enzyme is involved in an autosomal recessive disorders that impacts the catabolism of ketone bodies and isoleucine: beta-ketothiolase deficiency, leading to their deficiency within mitochondria. The mutation takes place within the acetoacetyl-CoA thiolase (ACAT) gene mapped on chromosome 11q22.3-23.1.[6]
Mutations in mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (HMG-CoA synthase) is another inherited autosomal recessive disorder affecting the catabolism of ketone bodies and can lead to the build-up of acetoacetyl-CoA.[7]
Additional application
[edit]Acetoacetyl-CoA also behaves as a product of acetoacetyl-CoA synthetase (AACS) within the cytosol, using acetoacetate as the substrate, the reaction provides acetyl groups for lipogenesis.[8] Understanding acetoacetyl-CoA is important in cholesterol development and lipogenesis and Acetoacetyl-CoA synthetase playing a role in its development, it also plays a significant role within the brain. Cholesterol and fats have been observed in high concentrations within neuronal tissue, as well as high AACS mRNA expression levels within cells of the hippocampus and cortical region. In addition, they play a significant role in neuronal development during the early embryonic and fetal developmental stages.[9]
See also
[edit]References
[edit]- ^ a b Hasegawa, Shinya; Noda, Kazuki; Maeda, Akina; Matsuoka, Masaru; Yamasaki, Masahiro; Fukui, Tetsuya (2012-11-01). "Acetoacetyl-CoA synthetase, a ketone body-utilizing enzyme, is controlled by SREBP-2 and affects serum cholesterol levels". Molecular Genetics and Metabolism. 107 (3): 553–560. doi:10.1016/j.ymgme.2012.08.017. ISSN 1096-7192. PMID 22985732.
- ^ Bruice PY (2017). Organic chemistry. Pearson. ISBN 978-0-13-404228-2. OCLC 974910578.
- ^ Tsuda H, Shiraki M, Inoue E, Saito T (August 2016). "Generation of poly-β-hydroxybutyrate from acetate in higher plants: Detection of acetoacetyl CoA reductase- and PHB synthase- activities in rice". Journal of Plant Physiology. 201: 9–16. Bibcode:2016JPPhy.201....9T. doi:10.1016/j.jplph.2016.06.007. PMID 27372278.
- ^ Matsumoto K, Tanaka Y, Watanabe T, Motohashi R, Ikeda K, Tobitani K, et al. (October 2013). "Directed evolution and structural analysis of NADPH-dependent Acetoacetyl Coenzyme A (Acetoacetyl-CoA) reductase from Ralstonia eutropha reveals two mutations responsible for enhanced kinetics". Applied and Environmental Microbiology. 79 (19): 6134–6139. Bibcode:2013ApEnM..79.6134M. doi:10.1128/aem.01768-13. PMC 3811355. PMID 23913421.
- ^ Taroncher-Oldenburg G, Nishina K, Stephanopoulos G (October 2000). "Identification and analysis of the polyhydroxyalkanoate-specific beta-ketothiolase and acetoacetyl coenzyme A reductase genes in the cyanobacterium Synechocystis sp. strain PCC6803". Applied and Environmental Microbiology. 66 (10): 4440–4448. Bibcode:2000ApEnM..66.4440T. doi:10.1128/aem.66.10.4440-4448.2000. PMC 92322. PMID 11010896.
- ^ Bissonnette, Bruno; Luginbuehl, Igor; Marciniak, Bruno; Dalens, Bernard J. (2006), "Mitochondrial Acetoacetyl-CoA Thiolase (ACAT) Deficiency", Syndromes: Rapid Recognition and Perioperative Implications, New York, NY: The McGraw-Hill Companies, retrieved 2022-12-08
- ^ Aledo, Rosa; Zschocke, Johannes; Pié, Juan; Mir, Cecilia; Fiesel, Sonja; Mayatepek, Ertan; Hoffmann, Georg F.; Casals, Núria; Hegardt, Fausto G. (2001-07-01). "Genetic basis of mitochondrial HMG-CoA synthase deficiency". Human Genetics. 109 (1): 19–23. doi:10.1007/s004390100554. ISSN 1432-1203. PMID 11479731. S2CID 24115345.
- ^ Hasegawa, Shinya; Ikeda, Yotaro; Yamasaki, Masahiro; Fukui, Tetsuya (2012). "The role of acetoacetyl-CoA synthetase, a ketone body-utilizing enzyme, in 3T3-L1 adipocyte differentiation". Biological & Pharmaceutical Bulletin. 35 (11): 1980–1985. doi:10.1248/bpb.b12-00435. ISSN 1347-5215. PMID 23123469.
- ^ Hasegawa, Shinya; Kume, Hiroki; Iinuma, Sayuri; Yamasaki, Masahiro; Takahashi, Noriko; Fukui, Tetsuya (2012-10-19). "Acetoacetyl-CoA synthetase is essential for normal neuronal development". Biochemical and Biophysical Research Communications. 427 (2): 398–403. doi:10.1016/j.bbrc.2012.09.076. ISSN 0006-291X. PMID 23000407.
