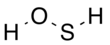Hydrogen thioperoxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sulfenic acid
| |||
| Systematic IUPAC name
Thioperoxol | |||
| Other names
Sulfenic acid
oxadisulfane Sulfur hydride hydroxide Sulfonol Sulfanol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| 672 | |||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H2OS | |||
| Molar mass | 50.08 g·mol−1 | ||
| Density | 1.249 | ||
Refractive index (nD)
|
1.484 | ||
| Related compounds | |||
Related compounds
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hydrogen thioperoxide, also called oxadisulfane or sulfanol, is the chemical with the structure H–S–O–H. It can be considered as the simple sulfur-substituted analog of the common hydrogen peroxide (H–O–O–H) chemical, and as the simplest hydrogen chalcogenide containing more than one type of chalcogen. The chemical has been described as the "missing link" between hydrogen peroxide and hydrogen disulfide (H–S–S–H),[2] though it is substantially less stable than either of the other two. It is the inorganic parent structure of the sulfenic acid class of organic compounds (R–S–O–H) and also the oxadisulfide linkage (R1–S–O–R2), where "R" is any organic structure. Sulfur is present in oxidation state 0.
Formation
[edit]Hydrogen thioperoxide has been synthesized in labs by photolysis of a mixture of ozone and hydrogen sulfide frozen in argon at 8 K[3] and by pyrolysis of di-tert-butyl sulfoxide.[2][4] Yet another synthesis is by an electric discharge through water and sulfur.[5]
In the interstellar medium there is a hypothesis that hydrogen thioperoxide is formed in a reaction of sulfur monoxide with the trihydrogen cation, dihydrogen and an electron. Another possible route, is sulfur monoxide reacting with atomic hydrogen to form HOS and HSO which in turn can add another hydrogen atom. However this mechanism probably needs a dust grain to take away excess energy.[6]
Properties
[edit]Hydrogen thioperoxide molecules have a gauche conformation.[7] They are unsymmetrical, but have a low barrier to convert from left-hand to right-hand forms, so that the molecule can tunnel between the forms.[5]
The measurements of the bond-lengths in hydrogen thioperoxide are H–S 1.3420 Å, S–O 1.6616 Å, O–H 0.9606 Å. The bond angles are H–S–O 98.57°, S–O–H 107.19°. The H–S and O–H bonds are twisted at 90.41°.[8]
The half-life of hydrogen thioperoxide in water is 40 minutes, much longer than the expected half-life of less than seconds for a sulfenic acid.[9]
Reactions
[edit]Two molecules of hydrogen thioperoxide can undergo cyclocondensation to form sulfinothioic acid HS(=O)SH and water.[10]
Hydrosulfide HS− can react with HSOH to yield disulfane HSSH.[11]
References
[edit]- ^ Iraqi, Muhammad; Schwarz, Helmut (April 1994). "Experimental evidence for the gas phase existence of HSOH (hydrogen thioperoxide) and SOH2 (thiooxonium ylide)". Chemical Physics Letters. 221 (5–6): 359–362. Bibcode:1994CPL...221..359I. doi:10.1016/0009-2614(94)00293-2.
- ^ a b Winnewisser, G.; Lewen, F.; Thorwirth, S.; Behnke, M.; Hahn, J.; Gauss, J.; Herbst, E. (2003). "Gas-Phase Detection of HSOH: Synthesis by Flash Vacuum Pyrolysis of Di-tert-butyl Sulfoxide and Rotational-Torsional Spectrum". Chem. Eur. J. 9 (22): 5501–5510. Bibcode:2003CEJ.....9.5501W. doi:10.1002/chem.200305192. PMID 14639633.
- ^ Smardzewski1, R.R.; Lin, M.C. (1977). "Matrix reactions of oxygen atoms with H2S molecules". J. Chem. Phys. 66 (7): 3197–3204. Bibcode:1977JChPh..66.3197S. doi:10.1063/1.434294.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Beckers, H.; Esser, S.; Metzroth, T.; Behnke, M.; Willner, H.; Gauss, J.; Hahn, J. (2006). "Low-Pressure Pyrolysis of tBu2SO: Synthesis and IR Spectroscopic Detection of HSOH". Chem. Eur. J. 12 (3): 832–844. doi:10.1002/chem.200500104. PMID 16240313.
- ^ a b Baum, Oliver (2008). HSOH: An Elusive Species with Many Different Traits (PDF). Cuvillier Verlag. pp. 1–2. ISBN 9783867277907. Archived from the original (PDF) on 2017-03-12. Retrieved 2017-03-11.
- ^ Baum 70-73
- ^ Cárdenas-Jirón, G.I.; Letelier, J.R.; Toro-Labbé, A. (1998). "The Internal Rotation of Hydrogen Thioperoxide: Energy, Chemical Potential, and Hardness Profiles". J. Phys. Chem. A. 102 (40): 7864–7871. Bibcode:1998JPCA..102.7864C. doi:10.1021/jp981841j.
- ^ Baum 84
- ^ Kumar, Murugaeson R.; Farmer, Patrick J. (2018). "Chemical trapping and characterization of small oxoacids of sulfur (SOS) generated in aqueous oxidations of H2S". Redox Biology. 14: 485–491. doi:10.1016/j.redox.2017.10.012. PMC 5680521. PMID 29096321.
- ^ Freeman, Fillmore; Bui, An; Dinh, Lauren; Hehre, Warren J. (2 August 2012). "Dehydrative Cyclocondensation Mechanisms of Hydrogen Thioperoxide and of Alkanesulfenic Acids". The Journal of Physical Chemistry A. 116 (30): 8031–8039. Bibcode:2012JPCA..116.8031F. doi:10.1021/jp3024827. PMID 22724673.
- ^ Kolloru, Gopi K. (25 February 2015). Hydrogen Sulfide in Redox Biology. Academic Press. p. 274. ISBN 9780128016237.