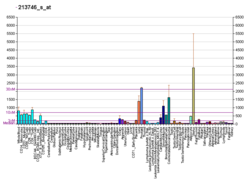
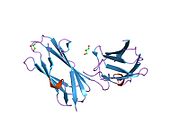
FLNA
Filamin A, alpha (FLNA) is a protein that in humans is encoded by the FLNA gene.[5][6]
Function
[edit]Actin-binding protein, or filamin, is a 280-kD protein that crosslinks actin filaments into orthogonal networks in cortical cytoplasm and participates in the anchoring of membrane proteins for the actin cytoskeleton. Remodeling of the cytoskeleton is central to the modulation of cell shape and migration. Filamin A, encoded by the FLNA gene, is a widely expressed filamin that regulates the reorganization of the actin cytoskeleton by interacting with integrins, transmembrane receptor complexes, and secondary messengers.[7] At least 31 disease-causing mutations in this gene have been discovered.[8]
Structure
[edit]The protein structure includes an actin binding N terminal domain, 24 internal repeats and 2 hinge regions.[9][10]
Interactions
[edit]Filamin has been shown to interact with:
RNA editing
[edit]The edited residue was previously recorded as a single nucleotide polymorphism(SNP) in dbSNP.
Type
[edit]A to I RNA editing is catalyzed by a family of adenosine deaminases acting on RNA (ADARs) that specifically recognize adenosines within double-stranded regions of pre-mRNAs and deaminate them to inosine. Inosines are recognised as guanosine by the cells translational machinery. There are three members of the ADAR family ADARs 1-3 with ADAR 1 and ADAR 2 being the only enzymatically active members.ADAR3 is thought to have a regulatory role in the brain. ADAR1 and ADAR 2 are widely expressed in tissues while ADAR 3 is restricted to the brain. The double stranded regions of RNA are formed by base-pairing between residues in a region complementary to the region of the editing site. This complementary region is usually found in a neighbouring intron but can also be located in an exonic sequence. The region that base pairs with the editing region is known as an Editing Complentary Sequence (ECS).
Site
[edit]The one editing site of FLNA pre-mRNA is located within amino acid 2341 of the final protein. The Glutamine (Q) codon is altered due to a site specific deamination of an adenosine at the editing site to an Arginine (R) codon. The editing region is predicted to form a double stranded region of 32 base pairs in length with a complementary sequence about 200 nucleotides downstream of the editing site. This ECS is found in an intronic sequence.[25] Editing at the Q/R site is likely to involve both ADAR1 and ADAR2.Mice ADAR2 knockouts show a decrease in editing at the Q/R site.ADAR1 double knockouts have no effect on editing.[26]
Structure
[edit]The edited adenosine is located in the 22 immunogloulin[check spelling] like repeat of the protein. This region is an integrin β binding domain[27] and a RAC1 binding domain.[20] The amino acid change is likely to effect the electrostatic potential of the binding domains.[25] FLNA editing site is 2 nucleotides from a splice site like the R/G site of GluR-2. Both transcripts have 7/8 identical nucleotides around their editing sites. Since it is widely thought that editing at the GLUR-2 Q/R site influences splicing, the sequence and editing site similarity could mean that editing at the FLNA site could also regulate splicing. In vitro experiments of gluR-2 have shown that presence of ADAR2 results in inhibition of splicing.[28] Analysis of EST data for FLNA show that there is a link between editing of the last exon codon and retention of the following intron.[25]
Function
[edit]The change in electrostatic potential is likely to effect the binding of FLNA to the many proteins it interacts with.[29]
DNA repair
[edit]Interaction of FLNA with the BRCA1 protein is required for efficient regulation of early stages of DNA repair processes.[30] FLNA is implicated in the control of the DNA repair process of homologous recombination and non-homologous end joining.[30]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000196924 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031328 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Gorlin JB, Henske E, Warren ST, Kunst CB, D'Urso M, Palmieri G, Hartwig JH, Bruns G, Kwiatkowski DJ (October 1993). "Actin-binding protein (ABP-280) filamin gene (FLN) maps telomeric to the color vision locus (R/GCP) and centromeric to G6PD in Xq28". Genomics. 17 (2): 496–8. doi:10.1006/geno.1993.1354. PMID 8406501.
- ^ Robertson SP, Twigg SR, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, Kenwrick SJ, Kim CA, Morava E, Newbury-Ecob R, Orstavik KH, Quarrell OW, Schwartz CE, Shears DJ, Suri M, Kendrick-Jones J, Wilkie AO (March 2003). "Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans". Nat Genet. 33 (4): 487–91. doi:10.1038/ng1119. PMID 12612583.
- ^ "Entrez Gene: FLNA filamin A, alpha (actin binding protein 280)".
- ^ Šimčíková D, Heneberg P (December 2019). "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases". Scientific Reports. 9 (1): 18577. Bibcode:2019NatSR...918577S. doi:10.1038/s41598-019-54976-4. PMC 6901466. PMID 31819097.
- ^ Gräber P, Witt HT (February 1976). "Relations between the electrical potential, pH gradient, proton flux and phosphorylation in the photosynthetic membrane". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 423 (2): 141–63. doi:10.1016/0005-2728(76)90174-2. PMID 2316.
- ^ "P21333 (FLNA_HUMAN): Filamin-A". UniProt.
- ^ Yuan Y, Shen Z (December 2001). "Interaction with BRCA2 suggests a role for filamin-1 (hsFLNa) in DNA damage response". J. Biol. Chem. 276 (51): 48318–24. doi:10.1074/jbc.M102557200. PMID 11602572.
- ^ van der Flier A, Kuikman I, Kramer D, Geerts D, Kreft M, Takafuta T, Shapiro SS, Sonnenberg A (January 2002). "Different splice variants of filamin-B affect myogenesis, subcellular distribution, and determine binding to integrin [beta] subunits". J. Cell Biol. 156 (2): 361–76. doi:10.1083/jcb.200103037. PMC 2199218. PMID 11807098.
- ^ Loo DT, Kanner SB, Aruffo A (September 1998). "Filamin binds to the cytoplasmic domain of the beta1-integrin. Identification of amino acids responsible for this interaction". J. Biol. Chem. 273 (36): 23304–12. doi:10.1074/jbc.273.36.23304. PMID 9722563.
- ^ Hjälm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM (September 2001). "Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase". J. Biol. Chem. 276 (37): 34880–7. doi:10.1074/jbc.M100784200. PMID 11390380.
- ^ Awata H, Huang C, Handlogten ME, Miller RT (September 2001). "Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein". J. Biol. Chem. 276 (37): 34871–9. doi:10.1074/jbc.M100775200. PMID 11390379.
- ^ Tu Y, Wu S, Shi X, Chen K, Wu C (April 2003). "Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation". Cell. 113 (1): 37–47. doi:10.1016/s0092-8674(03)00163-6. PMID 12679033.
- ^ Nagano T, Yoneda T, Hatanaka Y, Kubota C, Murakami F, Sato M (July 2002). "Filamin A-interacting protein (FILIP) regulates cortical cell migration out of the ventricular zone". Nat. Cell Biol. 4 (7): 495–501. doi:10.1038/ncb808. PMID 12055638. S2CID 4795393.
- ^ Sheen VL, Feng Y, Graham D, Takafuta T, Shapiro SS, Walsh CA (November 2002). "Filamin A and Filamin B are co-expressed within neurons during periods of neuronal migration and can physically interact". Hum. Mol. Genet. 11 (23): 2845–54. doi:10.1093/hmg/11.23.2845. PMID 12393796.
- ^ Donaldson JC, Dise RS, Ritchie MD, Hanks SK (August 2002). "Nephrocystin-conserved domains involved in targeting to epithelial cell-cell junctions, interaction with filamins, and establishing cell polarity". J. Biol. Chem. 277 (32): 29028–35. doi:10.1074/jbc.M111697200. PMID 12006559.
- ^ a b Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP (March 1999). "The small GTPase RalA targets filamin to induce filopodia". Proc. Natl. Acad. Sci. U.S.A. 96 (5): 2122–8. Bibcode:1999PNAS...96.2122O. doi:10.1073/pnas.96.5.2122. PMC 26747. PMID 10051605.
- ^ He X, Li Y, Schembri-King J, Jakes S, Hayashi J (August 2000). "Identification of actin binding protein, ABP-280, as a binding partner of human Lnk adaptor protein". Mol. Immunol. 37 (10): 603–12. doi:10.1016/s0161-5890(00)00070-5. PMID 11163396.
- ^ Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A (December 2000). "The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin". Nat. Cell Biol. 2 (12): 888–92. doi:10.1038/35046533. PMID 11146652. S2CID 10182923.
- ^ Tsuchiya H, Iseda T, Hino O (July 1996). "Identification of a novel protein (VBP-1) binding to the von Hippel-Lindau (VHL) tumor suppressor gene product". Cancer Res. 56 (13): 2881–5. PMID 8674032.
- ^ Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C, Cohen HT (October 2002). "The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1". J. Biol. Chem. 277 (42): 39887–98. doi:10.1074/jbc.M205040200. PMID 12169691.
- ^ a b c Levanon EY, Hallegger M, Kinar Y, Shemesh R, Djinovic-Carugo K, Rechavi G, Jantsch MF, Eisenberg E (2005). "Evolutionarily conserved human targets of adenosine to inosine RNA editing". Nucleic Acids Res. 33 (4): 1162–8. arXiv:q-bio/0502045. Bibcode:2005q.bio.....2045L. doi:10.1093/nar/gki239. PMC 549564. PMID 15731336.
- ^ Riedmann EM, Schopoff S, Hartner JC, Jantsch MF (June 2008). "Specificity of ADAR-mediated RNA editing in newly identified targets". RNA. 14 (6): 1110–8. doi:10.1261/rna.923308. PMC 2390793. PMID 18430892.
- ^ Travis MA, van der Flier A, Kammerer RA, Mould AP, Sonnenberg A, Humphries MJ (July 2004). "Interaction of filamin A with the integrin beta 7 cytoplasmic domain: role of alternative splicing and phosphorylation". FEBS Lett. 569 (1–3): 185–90. Bibcode:2004FEBSL.569..185T. doi:10.1016/j.febslet.2004.04.099. PMID 15225631.
- ^ Bratt E, Ohman M (March 2003). "Coordination of editing and splicing of glutamate receptor pre-mRNA". RNA. 9 (3): 309–18. doi:10.1261/rna.2750803. PMC 1370398. PMID 12592005.
- ^ Popowicz GM, Müller R, Noegel AA, Schleicher M, Huber R, Holak TA (October 2004). "Molecular structure of the rod domain of dictyostelium filamin". J. Mol. Biol. 342 (5): 1637–46. doi:10.1016/j.jmb.2004.08.017. PMID 15364587.
- ^ a b Velkova A, Carvalho MA, Johnson JO, Tavtigian SV, Monteiro AN (April 2010). "Identification of Filamin A as a BRCA1-interacting protein required for efficient DNA repair". Cell Cycle. 9 (7): 1421–33. doi:10.4161/cc.9.7.11256. PMC 3040726. PMID 20305393.
Further reading
[edit]- Light S, Sagit R, Ithychanda SS, Qin J, Elofsson A (Sep 2012). "The evolution of filamin — a protein domain repeat perspective". Journal of Structural Biology. 179 (3): 289–98. doi:10.1016/j.jsb.2012.02.010. PMC 3728663. PMID 22414427.
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS (2001). "Filamins as integrators of cell mechanics and signalling". Nat. Rev. Mol. Cell Biol. 2 (2): 138–45. doi:10.1038/35052082. PMID 11252955. S2CID 5203942.
- van der Flier A, Sonnenberg A (2001). "Structural and functional aspects of filamins". Biochim. Biophys. Acta. 1538 (2–3): 99–117. doi:10.1016/S0167-4889(01)00072-6. PMID 11336782.
- Robertson, S. (October 2019). X-Linked Otopalatodigital Spectrum Disorders. GeneReviews® [Internet]. University of Washington, Seattle. PMID 20301567. NBK1393.
- Chen, M.H.; Walsh, C.A. (September 2021). FLNA Deficiency. GeneReviews® [Internet]. University of Washington, Seattle. PMID 20301392. NBK1213.