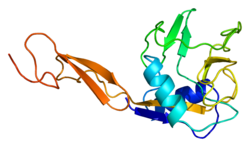E-selectin
E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a selectin cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the SELE gene.[5]
Structure
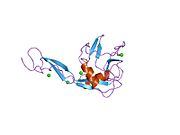
[edit]E selectin has a cassette structure: an N-terminal, C-type lectin domain, an EGF (epidermal-growth-factor)-like domain, 6 Sushi domain (SCR repeat) units, a transmembrane domain (TM) and an intracellular cytoplasmic tail (cyto). The three-dimensional structure of the ligand-binding region of human E-selectin has been determined at 2.0 Å resolution in 1994.[6] The structure reveals limited contact between the two domains and a coordination of Ca2+ not predicted from other C-type lectins. Structure/function analysis indicates a defined region and specific amino-acid side chains that may be involved in ligand binding. The E-selectin bound to sialyl-LewisX (SLeX; NeuNAcα2,3Galβ1,4[Fucα1,3]GlcNAc) tetrasaccharide was solved in 2000.[7]
Gene and regulation
[edit]In humans, E-selectin is encoded by the SELE gene. Its C-type lectin domain, EGF-like, SCR repeats, and transmembrane domains are each encoded by separate exons, whereas the E-selectin cytosolic domain derives from two exons. The E-selectin locus flanks the L-selectin locus on chromosome 1.[8]
Different from P-selectin, which is stored in vesicles called Weibel-Palade bodies, E-selectin is not stored in the cell and has to be transcribed, translated, and transported to the cell surface. The production of E-selectin is stimulated by the expression of P-selectin which in turn, is stimulated by tumor necrosis factor α (TNFα), interleukin-1 (IL-1) and lipopolysaccharide (LPS).[9][10] It takes about two hours, after cytokine recognition, for E-selectin to be expressed on the endothelial cell's surface. Maximal expression of E-selectin occurs around 6–12 hours after cytokine stimulation, and levels returns to baseline within 24 hours.[10]
Shear forces are also found to affect E-selectin expression. A high laminar shear enhances acute endothelial cell response to interleukin-1β in naïve or shear-conditioned endothelial cells as may be found in the pathological setting of ischemia/reperfusion injury while conferring rapid E-selectin down regulation to protect against chronic inflammation.[11]
Phytoestrogens, plant compounds with estrogen-like biological activity, such as genistein, formononetin, biochanin A and daidzein, as well as a mixture of these phytoestrogens were found able to reduce E-selectin as well as VCAM-1 and ICAM-1 on cell surface and in culture supernatant.[12]
Ligands
[edit]E-selectin recognizes and binds to sialylated carbohydrates present on the surface proteins of certain leukocytes. E-selectin ligands are expressed by neutrophils, monocytes, eosinophils, memory-effector T-like lymphocytes, and natural killer cells. Each of these cell types is found in acute and chronic inflammatory sites in association with expression of E-selectin, thus implicating E-selectin in the recruitment of these cells to such inflammatory sites.
These carbohydrates include members of the Lewis X and Lewis A families found on monocytes, granulocytes, and T-lymphocytes.[13]
The glycoprotein ESL-1, present on neutrophils and myeloid cells, was the first counter-receptor for E-selectin to be described. It is a variant of the tyrosine kinase FGF glycoreceptor, raising the possibility that its binding to E-selectin is involved in initiating signaling in the bound cells.
P-selectin glycoprotein ligand-1 (PSGL-1) derived from human neutrophils is also a high-efficiency ligand for endothelium-expressed E-selectin under flow.[14] It mediates the rolling of leukocytes on the activated endothelium surrounding an inflamed tissue.
Both ESL-1 and PSGL-1 should bear sialyl Lewis a/x in order to bind E/P-selectins.[15]
E-selectin is found to mediate the adhesion of tumor cells to endothelial cells, by binding to E-selectin ligands on the tumor cells. E-selectin ligands also play a role in cancer metastasis. The role of these two E-selectin ligands in metastasis in vivo is poorly defined and remains to be firmly demonstrated. PSGL-1 was detected on the surfaces of bone-metastatic prostate tumor cells, suggesting that it may have a functional role in the bone tropism of prostate tumor cells.[16]
In cancer cells, CD44, death receptor-3 (DR3), LAMP1, and LAMP2 were identified as E-selectin ligands present on colon cancer cells,[17] and CD44v, Mac2-BP, and gangliosides were identified as E-selectin ligands present on breast cancer cells.[18][19][20]
On human neutrophils the glycosphingolipid NeuAcα2-3Galβ1-4GlcNAcβ1-3[Galβ1-4(Fucα1-3)GlcNAcβ1-3]2[Galβ1-4GlcNAcβ1-3]2Galβ1-4GlcβCer (and closely related structures) are functional E-selectin receptors.[21]
Function
[edit]Role in inflammation
[edit]During inflammation, E-selectin plays an important part in recruiting leukocytes to the site of injury. The local release of cytokines IL-1 and TNF-α by macrophages in the inflamed tissue induces the over-expression of E-selectin on endothelial cells of nearby blood vessels.[22] Leukocytes in the blood expressing the correct ligand will bind with low affinity to E-selectin, also under the shear stress of blood flow, causing the leukocytes to "roll" along the internal surface of the blood vessel as temporary interactions are made and broken.
As the inflammatory response progresses, chemokines released by injured tissue enter the blood vessels and activate the rolling leukocytes, which are now able to tightly bind to the endothelial surface and begin making their way into the tissue.[13]
P-selectin has a similar function, but is expressed on the endothelial cell surface within minutes as it is stored within the cell rather than produced on demand.[13]
Role in cancer
[edit]E-selectin was first discovered as an transmembrane receptor induced in endothelial cells upon inflammatory stimulation which mediated adhesion of monocytic or HL60 leukemic cells.[23][24] This led to the hypothesis that cancer cells secreted inflammatory cytokines such as IL-1β or TNFα to induce E-selectin at distant metastatic sites. This induction would enable circulating tumor cells to arrest at stimulated sites, roll along activated endothelium, extravasate, and form metastases.[25] Studies since have showed that E-selectin binding to colon cancer cells correlates with increasing metastatic potential,[26] and that cancer cells of multiple tumor types bind E-selectin using glycoprotein or glycolipid ligands normally expressed on immune cells.[27][28] Studies have further described a mechanistic cascade wherein cancer cells first bind E-selectin at shear flow rates: E-selectin binding results in a velcro-like interaction allowing the cancer cells to engage higher affinity integrin binding that eventually results in a tight binding between tumor cells and the activated endothelium.[29][30]
While numerous pieces of in vitro and clinical evidence continue to support this hypothesis of E-selectin-mediated cancer metastasis, in vivo studies of cancer metastasis have shown that E-selectin knockout only minimally affects leukemic cell adhesion to bone immediately following injection.[31] while experimental lung metastasis is not affected by the genetic deletion of E-selectin.[32][33] Furthermore, studies have also shown that primary tumor growth is increased in E-selectin knockout mice.[34][35] This paradox was more recently solved by a trio of studies showing that E-selectin is only constitutively expressed in the bone marrow endothelium[36] where it is thought to perform functions vital to hematopoiesis.[37] that are hijacked specifically by cells metastasizing to bone and not other sites.[38] This data supports ongoing clinical efforts to inhibit breast cancer bone metastasis with E-selectin-blocking agents.[39] The complexity of E-selectin ligand biology may also play a role in these discrepant in vitro and in vivo results. At least 15 different glycoprotein and glycolipid substrates for E-selectin have been described on various cancer cells, while only n-glycan Glg1 (Esl1) was shown to mediate bone metastasis.[40] Other ligands or combinations thereof may result in distinct mechanisms during cancer metastasis.
Beyond a direct interaction with tumor cells, E-selectin induction in response to cytokines locally secreted by cancer cells enables specific tumor targeting of sLeX-conjugated nanoparticles or thioaptamers containing anti-tumor payloads.[41] In addition, E-selectin may also function to recruit monocytes to primary tumors or lung metastases to promote an inflammatory pro-tumor microenvironment.[42] Blocking these interactions or enabling trafficking of CAR-T cells to E-selectin-positive sites may hold promise for future therapeutic development.
Pathological relevance
[edit]Critical illness polyneuromyopathy
[edit]In cases of elevated blood glucose levels, such as in sepsis, E-selectin expression is higher than normal, resulting in greater microvascular permeability. The greater permeability leads to edema (swelling) of the skeletal endothelium (blood vessel linings), resulting in skeletal muscle ischemia (restricted blood supply) and eventually necrosis (cell death). This underlying pathology is the cause of the symptomatic disease critical illness polyneuromyopathy (CIPNM).[43] Traditional Chinese herbal medicines, like berberine downregulate E-selectin.[44]
Pathogen attachment
[edit]Study shows the adherence of Porphyromonas gingivalis to human umbilical vein endothelial cells increases with the induction of E-selectin expression by TNF-α. An antibody to E-selectin and sialyl LewisX suppressed P. gingivalis adherence to stimulated HUVECs. P. gingivalis mutants lacking OmpA-like proteins Pgm6/7 had reduced adherence to stimulated HUVECs, but fimbriae-deficient mutants were not affected. E-selecin-mediated P. gingivalis adherence activated endothelial exocytosis. These results suggest that the interaction between host E-selectin and pathogen Pgm6/7 mediates P. gingivalis adherence to endothelial cells and may trigger vascular inflammation.[45]
Acute coronary syndrome
[edit]The immunohistochemical expressions of E-selectin and PECAM-1 were significantly increased at intima in vulnerable plaques of acute coronary syndrome (ACS) group, especially in neovascular endothelial cells, and positively correlated with inflammatory cell density, suggesting that PECAM-1 and E-selectin might play an important role in inflammatory reaction and development of vulnerable plaque. E-selectin Ser128Arg polymorphism is associated with ACS, and it might be a risk factor for ACS.[46]
Nicotine-mediated induction
[edit]Smoking is highly correlated with enhanced likelihood of atherosclerosis by inducing endothelial dysfunction. In endothelial cells, various cell-adhesion molecules including E-selectin, are shown to be upregulated upon exposure to nicotine, the addictive component of tobacco smoke. Nicotine-stimulated adhesion of monocytes to endothelial cells is dependent on the activation of α7-nAChRs, β-Arr1 and cSrc regulated increase in E2F1-mediated transcription of E-selectin gene. Therefore, agents such as RRD-251 that can target activity of E2F1 may have potential therapeutic benefit against cigarette smoke induced atherosclerosis.[47]
Cerebral aneurysm
[edit]It's also found that E-selectin expression increased in human ruptured cerebral aneurysm tissues. E-selectin might be an important factor involved in the process of cerebral aneurysm formation and rupture, by promoting inflammation and weakening cerebral artery walls.[48]
As a biomarker
[edit]E-selectin is also an emerging biomarker for the metastatic potential of some cancers including colorectal cancer and recurrences.[49]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000007908 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000026582 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Collins T, Williams A, Johnston GI, Kim J, Eddy R, Shows T, et al. (February 1991). "Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1". The Journal of Biological Chemistry. 266 (4): 2466–73. doi:10.1016/S0021-9258(18)52267-5. PMID 1703529.
- ^ Graves BJ, Crowther RL, Chandran C, Rumberger JM, Li S, Huang KS, et al. (February 1994). "Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains". Nature. 367 (6463): 532–8. Bibcode:1994Natur.367..532G. doi:10.1038/367532a0. PMID 7509040. S2CID 4338500.
- ^ Somers WS, Tang J, Shaw GD, Camphausen RT (October 2000). "Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1". Cell. 103 (3): 467–79. doi:10.1016/S0092-8674(00)00138-0. PMID 11081633. S2CID 12719907.
- ^ Cummings RD (2008). "Selectins". In Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (eds.). Essentials of Glycobiology (2nd ed.). Plainview, N.Y: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-770-9.
- ^ Janeway C (2005). Immunobiology: the immune system in health and disease. New York: Garland Science. ISBN 0-8153-4101-6.
- ^ a b Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, et al. (December 1992). "E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro". Immunology. 77 (4): 543–9. PMC 1421640. PMID 1283598.
- ^ Huang RB, Eniola-Adefeso O (2012). "Shear stress modulation of IL-1β-induced E-selectin expression in human endothelial cells". PLOS ONE. 7 (2): e31874. Bibcode:2012PLoSO...731874H. doi:10.1371/journal.pone.0031874. PMC 3286450. PMID 22384091.
- ^ Andrade CM, Sá MF, Toloi MR (April 2012). "Effects of phytoestrogens derived from soy bean on expression of adhesion molecules on HUVEC". Climacteric. 15 (2): 186–94. doi:10.3109/13697137.2011.582970. PMID 22066752. S2CID 29123853.
- ^ a b c Robbins SL, Cotran RS, Kumar V, Collins T (1999). Robbins pathologic basis of disease. Philadelphia: WB Saunders. ISBN 0-7216-7335-X.
- ^ Zou X, Shinde Patil VR, Dagia NM, Smith LA, Wargo MJ, Interliggi KA, et al. (August 2005). "PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow". American Journal of Physiology. Cell Physiology. 289 (2): C415-24. doi:10.1152/ajpcell.00289.2004. PMID 15814589.
- ^ Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N (May 2004). "Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis". Cancer Science. 95 (5): 377–84. doi:10.1111/j.1349-7006.2004.tb03219.x. PMC 11159147. PMID 15132763. S2CID 27761640.
- ^ Dimitroff CJ, Descheny L, Trujillo N, Kim R, Nguyen V, Huang W, et al. (July 2005). "Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells". Cancer Research. 65 (13): 5750–60. doi:10.1158/0008-5472.CAN-04-4653. PMC 1472661. PMID 15994950.
- ^ Gout S, Tremblay PL, Huot J (2008). "Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis". Clinical & Experimental Metastasis. 25 (4): 335–44. doi:10.1007/s10585-007-9096-4. PMID 17891461. S2CID 11272581.
- ^ Shirure VS, Liu T, Delgadillo LF, Cuckler CM, Tees DF, Benencia F, et al. (January 2015). "CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions". American Journal of Physiology. Cell Physiology. 308 (1): C68-78. doi:10.1152/ajpcell.00094.2014. PMC 4281670. PMID 25339657.
- ^ Shirure VS, Reynolds NM, Burdick MM (2012). "Mac-2 binding protein is a novel E-selectin ligand expressed by breast cancer cells". PLOS ONE. 7 (9): e44529. Bibcode:2012PLoSO...744529S. doi:10.1371/journal.pone.0044529. PMC 3435295. PMID 22970241.
- ^ Shirure VS, Henson KA, Schnaar RL, Nimrichter L, Burdick MM (March 2011). "Gangliosides expressed on breast cancer cells are E-selectin ligands". Biochemical and Biophysical Research Communications. 406 (3): 423–9. doi:10.1016/j.bbrc.2011.02.061. PMID 21329670.
- ^ Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, et al. (November 2008). "E-selectin receptors on human leukocytes". Blood. 112 (9): 3744–52. doi:10.1182/blood-2008-04-149641. PMC 2572800. PMID 18579791.
- ^ Janeway's Immunobiology, 8th edition: "pattern recognition by cells of the innate immunity system", chapter 3 page 83.
- ^ Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA (December 1987). "Identification of an inducible endothelial-leukocyte adhesion molecule". Proceedings of the National Academy of Sciences of the United States of America. 84 (24): 9238–42. Bibcode:1987PNAS...84.9238B. doi:10.1073/pnas.84.24.9238. PMC 299728. PMID 2827173.
- ^ Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B (November 1990). "Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells". Science. 250 (4984): 1132–5. Bibcode:1990Sci...250.1132W. doi:10.1126/science.1701275. PMID 1701275.
- ^ Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P (March 1999). "Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells". Cancer Research. 59 (6): 1356–61. PMID 10096570.
- ^ Sawada R, Tsuboi S, Fukuda M (January 1994). "Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials". The Journal of Biological Chemistry. 269 (2): 1425–31. doi:10.1016/S0021-9258(17)42275-7. PMID 7507108.
- ^ Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL (August 2004). "Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin". Cancer Research. 64 (15): 5261–9. doi:10.1158/0008-5472.CAN-04-0691. PMID 15289332. S2CID 11632075.
- ^ Laferrière J, Houle F, Huot J (2004). "Adhesion of HT-29 colon carcinoma cells to endothelial cells requires sequential events involving E-selectin and integrin beta4". Clinical & Experimental Metastasis. 21 (3): 257–64. doi:10.1023/B:CLIN.0000037708.09420.9a. PMID 15387376. S2CID 22354589.
- ^ Barthel SR, Hays DL, Yazawa EM, Opperman M, Walley KC, Nimrichter L, et al. (January 2013). "Definition of molecular determinants of prostate cancer cell bone extravasation". Cancer Research. 73 (2): 942–52. doi:10.1158/0008-5472.CAN-12-3264. PMC 3548951. PMID 23149920.
- ^ Esposito M, Kang Y (February 2014). "Targeting tumor-stromal interactions in bone metastasis". Pharmacology & Therapeutics. 141 (2): 222–33. doi:10.1016/j.pharmthera.2013.10.006. PMC 3947254. PMID 24140083.
- ^ Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, et al. (June 2005). "In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment". Nature. 435 (7044): 969–73. Bibcode:2005Natur.435..969S. doi:10.1038/nature03703. PMC 2570168. PMID 15959517.
- ^ Läubli H, Borsig L (February 2010). "Selectins as mediators of lung metastasis". Cancer Microenvironment. 3 (1): 97–105. doi:10.1007/s12307-010-0043-6. PMC 2990482. PMID 21209777.
- ^ Esposito M, Mondal N, Greco TM, Wei Y, Spadazzi C, Lin SC, et al. (May 2019). "Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis". Nature Cell Biology. 21 (5): 627–639. doi:10.1038/s41556-019-0309-2. PMC 6556210. PMID 30988423.
- ^ Esposito M, Mondal N, Greco TM, Wei Y, Spadazzi C, Lin SC, et al. (May 2019). "Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis". Nature Cell Biology. 21 (5): 627–639. doi:10.1038/s41556-019-0309-2. PMC 6556210. PMID 30988423.
- ^ Taverna D, Moher H, Crowley D, Borsig L, Varki A, Hynes RO (January 2004). "Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins". Proceedings of the National Academy of Sciences of the United States of America. 101 (3): 763–8. Bibcode:2004PNAS..101..763T. doi:10.1073/pnas.0307289101. PMC 321755. PMID 14718670.
- ^ Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, et al. (May 2016). "Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone". Science Translational Medicine. 8 (340): 340ra73. doi:10.1126/scitranslmed.aad4059. PMC 8722465. PMID 27225183. S2CID 31317455.
- ^ Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, et al. (November 2012). "Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance". Nature Medicine. 18 (11): 1651–7. doi:10.1038/nm.2969. PMID 23086476. S2CID 5593456.
- ^ Esposito M, Mondal N, Greco TM, Wei Y, Spadazzi C, Lin SC, et al. (May 2019). "Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis". Nature Cell Biology. 21 (5): 627–639. doi:10.1038/s41556-019-0309-2. PMC 6556210. PMID 30988423.
- ^ "GlycoMimetics Announces Plans to Initiate Breast Cancer Trial to Evaluate GMI-1359". www.businesswire.com. 2019-04-12. Retrieved 2019-06-10.
- ^ Esposito M, Mondal N, Greco TM, Wei Y, Spadazzi C, Lin SC, et al. (May 2019). "Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis". Nature Cell Biology. 21 (5): 627–639. doi:10.1038/s41556-019-0309-2. PMC 6556210. PMID 30988423.
- ^ Mai J, Huang Y, Mu C, Zhang G, Xu R, Guo X, et al. (August 2014). "Bone marrow endothelium-targeted therapeutics for metastatic breast cancer". Journal of Controlled Release. 187: 22–9. doi:10.1016/j.jconrel.2014.04.057. PMC 4109393. PMID 24818768.
- ^ Läubli H, Spanaus KS, Borsig L (November 2009). "Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes". Blood. 114 (20): 4583–91. doi:10.1182/blood-2008-10-186585. PMID 19779041.
- ^ Visser LH (November 2006). "Critical illness polyneuropathy and myopathy: clinical features, risk factors and prognosis". European Journal of Neurology. 13 (11): 1203–12. doi:10.1111/j.1468-1331.2006.01498.x. PMID 17038033. S2CID 7557453.
- ^ Hu Y, Chen X, Duan H, Hu Y, Mu X (2009). "Chinese herbal medicinal ingredients inhibit secretion of IL-6, IL-8, E-selectin and TXB2 in LPS-induced rat intestinal microvascular endothelial cells". Immunopharmacology and Immunotoxicology. 31 (4): 550–5. doi:10.3109/08923970902814129. PMID 19874221. S2CID 207481204.
- ^ Komatsu T, Nagano K, Sugiura S, Hagiwara M, Tanigawa N, Abiko Y, et al. (July 2012). "E-selectin mediates Porphyromonas gingivalis adherence to human endothelial cells". Infection and Immunity. 80 (7): 2570–6. doi:10.1128/IAI.06098-11. PMC 3416463. PMID 22508864.
- ^ Fang F, Zhang W, Yang L, Wang Z, Liu DG (December 2011). "[PECAM-1 and E-selectin expression in vulnerable plague and their relationships to myocardial Leu125Val polymorphism of PECAM-1 and Ser128Arg polymorphism of E-selectin in patients with acute coronary syndrome]". Zhonghua Xin Xue Guan Bing Za Zhi (in Chinese). 39 (12): 1110–6. PMID 22336504.
- ^ Alamanda V, Singh S, Lawrence NJ, Chellappan SP (February 2012). "Nicotine-mediated induction of E-selectin in aortic endothelial cells requires Src kinase and E2F1 transcriptional activity". Biochemical and Biophysical Research Communications. 418 (1): 56–61. doi:10.1016/j.bbrc.2011.12.127. PMC 3273677. PMID 22240023.
- ^ Jia W, Wang R, Zhao J, Liu IY, Zhang D, Wang X, et al. (November 2011). "E-selectin expression increased in human ruptured cerebral aneurysm tissues". The Canadian Journal of Neurological Sciences. 38 (6): 858–62. doi:10.1017/s0317167100012439. PMID 22030423.
- ^ Sato H, Usuda N, Kuroda M, Hashimoto S, Maruta M, Maeda K (November 2010). "Significance of serum concentrations of E-selectin and CA19-9 in the prognosis of colorectal cancer". Japanese Journal of Clinical Oncology. 40 (11): 1073–80. doi:10.1093/jjco/hyq095. PMID 20576794.
External links
[edit]- E-Selectin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)