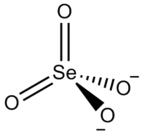
Zirconium selenate
Appearance
(Redirected from Zirconium(IV) selenate)
| |||
| |||
| Identifiers | |||
|---|---|---|---|
| |||
3D model (JSmol)
|
| ||
PubChem CID
|
| ||
| |||
| |||
| Properties | |||
| Zr(SeO4)2 | |||
| Appearance | colourless crystals (tetrahydrate)[1] | ||
| Density | 3.806 g·cm−3 (monohydrate)[1] 3.36 g·cm−3 (tetrahydrate) [2] | ||
| Boiling point | 580 °C (dec.)[3] | ||
| soluble (tetrahydrate) | |||
| Related compounds | |||
Other anions
|
zirconium sulfate | ||
Other cations
|
hafnium selenate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Zirconium selenate is an inorganic compound with the chemical formula Zr(SeO4)2. Its tetrahydrate can be obtained by the reaction of selenic acid and a saturated aqueous solution of zirconium oxychloride octahydrate (or zirconium hydroxide[2]). The tetrahydrate belongs to the orthorhombic crystal system and is isostructural with Zr(SO4)2·4H2O. It loses water when heated and becomes anhydrous at 220-230 °C.[3] It reacts with potassium fluoride to obtain K2Zr(SeO4)2F2·3H2O.[4]
References
[edit]- ^ a b Gerald Giester, Manfred Wildner (Aug 2018). "Contributions to the stereochemistry of zirconium oxysalts—part I: syntheses and crystal structures of novel Zr(SeO4)2·H2O and Zr(SeO4)2·4H2O". Monatshefte für Chemie - Chemical Monthly. 149 (8): 1321–1325. doi:10.1007/s00706-018-2226-7. ISSN 0026-9247. S2CID 103217478. Retrieved 2020-11-28.
- ^ a b M. A. Nabar, V. R. Ajgaonkar (1978-02-01). "Studies on selenates. III. Crystal chemical data for zirconium and cerium selenate tetrahydrates". Journal of Applied Crystallography. 11 (1): 56–57. Bibcode:1978JApCr..11...56N. doi:10.1107/S0021889878012686. ISSN 0021-8898. Archived from the original on 2018-06-03. Retrieved 2020-11-28.
- ^ a b Davidovich, R. L.; Medkov, M. A. Zirconium and hafnium diselenates. Koordinatsionnaya Khimiya, 1975. (1) 11: 1478-1480. ISSN 0132-344X.
- ^ Davidovich, R. L.; Medkov, M. A. Selenatofluoride complex compounds of zirconium and hafnium. Koordinatsionnaya Khimiya, 1975. 1 (12): 1646-1653. ISSN 0132-344X.

