Tris(acetylacetonato)cobalt(III)
| |
| Names | |
|---|---|
| Other names
Cobalt(III) acetylacetonate, tris(acac) cobalt
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.040.464 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H21CoO6 | |
| Molar mass | 356.260 g·mol−1 |
| Appearance | green solid |
| Density | 1.41 g/cm3 |
| Melting point | 213 °C (415 °F; 486 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H317, H334 | |
| P261, P264, P270, P272, P280, P285, P301+P312, P302+P352, P304+P341, P321, P330, P333+P313, P342+P311, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tris(acetylacetonato)cobalt(III) is the coordination complex with the formula Co(C5H7O2)3. Often abbreviated Co(acac)3, it is a green, diamagnetic solid that is soluble in organic solvents, but not in water. Owing to its solubility in organic solvents, tris(acetylacetonato)cobalt(III) is used to produce homogeneous catalysts by reduction.[1]
Structure
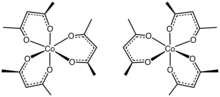
[edit]The structure of the complex has been confirmed by X-ray crystallography. The three acac− ligands bind in a bidentate fashion to cobalt, defining an octahedral complex.[2] The solid is isomorphous with tris(acetylacetonato)iron(III), tris(acetylacetonato)manganese(III), and tris(acetylacetonato)aluminium. With D3-symmetry, these complexes are chiral and often can be resolved into the individual enantiomers.
Synthesis and reactions
[edit]Tris(acetylacetonato)cobalt(III) is prepared by the reaction of cobalt(II) carbonate and acetylacetone in the presence of hydrogen peroxide:[3]
- 2 CoCO3 + 6 CH3COCH2COCH3 + H2O2 → 2 Co(O2C3Me2H)3 + 2 CO2 + 4 H2O
One distinctive aspect of Co(acac)3 is its susceptibility toward electrophilic aromatic substitution, by which protons on the central carbon are replaced with diverse electrophiles (Me = methyl):[4]
- Co(O2C3Me2H)3 + 3 NO2+ → Co(O2C3Me2NO2)3 + 3 H+
References
[edit]- ^ Mayo, Peter D.; Tam, William (2002). "Tris(acetoacetonyl)cobalt". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00084. ISBN 0-471-93623-5.
- ^ Arslan, Evrim; Lalancette, Roger A.; Bernal, Ivan (2017). "An Historic and Scientific Study of the Properties of Metal(III) Tris-acetylacetonates". Structural Chemistry. 28: 201–212. doi:10.1007/s11224-016-0864-0. S2CID 99668641.
- ^ Bryant, Burl E.; Fernelius, W. Conard (1957). Cobalt(III) Acetylacetonate. Inorganic Syntheses. Vol. 5. pp. 188–189. doi:10.1002/9780470132364.ch53. ISBN 978-0-470-13236-4.
- ^ Shalhoub, George M. (1980). "Co(acac)3 Synthesis, Reactions, and Spectra: An Experiment for General Chemistry". Journal of Chemical Education. 57 (7): 525. Bibcode:1980JChEd..57..525S. doi:10.1021/ed057p525.
