Aluminium acetylacetonate
Appearance
(Redirected from Tris(acetylacetonato)aluminium)

| |
| |
| Names | |
|---|---|
| IUPAC name
Tris(acetylacetonato)aluminium
| |
| Other names
Aluminium acetylacetonate, aluminium acetylacetonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.296 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Al(C5H7O2)3 | |
| Molar mass | 324.31 g/mol |
| Appearance | White solid[1] |
| Density | 1.42 g/cm3 |
| Melting point | 190-193 °C |
| Boiling point | 315 °C |
| Low | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
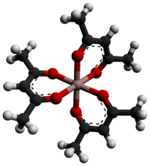
Aluminium acetylacetonate, also referred to as Al(acac)3, is a coordination complex with formula Al(C5H7O2)3. This aluminium complex with three acetylacetone ligands is used in research on Al-containing materials. The molecule has D3 symmetry, being isomorphous with other octahedral tris(acetylacetonate)s.[2]
Uses
[edit]Aluminium acetylacetonate can be used as the precursor to crystalline aluminium oxide films using low-pressure metal organic chemical vapour deposition.[3] In horticulture it can also be used as a molluscicide.[4]
References
[edit]- ^ Aluminium acetylacetonate
- ^ Dymock, K.; Palenik, Gus J. "Tris(acetylacetonato)gallium(III)" Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry (1974), volume 30, 1364-6. doi:10.1107/S0567740874004833. (this paper also discusses the Al compound)
- ^ "Carbonaceous alumina films deposited by MOCVD from aluminium acetylacetonate: a spectroscopic ellipsometry study"
- ^ I. F. Henderson, A. P. Martin (1990). "Control of slugs with contact-action molluscicides". Annals of Applied Biology. 116 (2): 273–278. doi:10.1111/j.1744-7348.1990.tb06607.x.
