Talk:Dry ice/Archive 1
| This is an archive of past discussions about Dry ice. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 |
Merge with carbon dioxide proposal
I agree we should move the physical properties out to the CO2 article. Dry ice, however, is unique amongst solids in that most people think about it separately from the gas. Thus I think it should stay as a separate article. Samw 23:52, 18 October 2005 (UTC)
- That is fine with me. It was the mismatched physical properties that I did not like. Feel free to do whatever you think will improve the article. Bobblewik 18:06, 19 October 2005 (UTC)
It should be mentioned that Dry Ice is also a very good store...
This should definitely be merged with the carbon dioxide article. That's all dry ice is - carbon dioxide. —Preceding unsigned comment added by Domanator (talk) 18:39, 20 September 2009 (UTC)
- The properties box has already been moved as per Samw's suggestion. There's plenty worth writing about dry ice, as this article demonstrates. Merging the whole article with carbon dioxide would either produce an article that is too long or require much useful information being discarded. It makes sense to have a separate article that details further information about a particular form of carbon dioxide.
- — Posted by Luke Goodsell, 21:52, 20 September 2009 (UTC)
Genericized trademark?
If dry ice is a genericized trademark, who once owned the name? -- stillnotelf 21:50, 22 July 2007 (UTC)
Trademarked by the Dry Ice Corporation of America in 1925.2A00:23C0:5100:A901:E0A6:9008:97BB:DEF6 (talk) 09:46, 2 December 2020 (UTC)
If you allow it to warm up, but keep it in a sealed container, it may change into a liquid state. Your container would need to be very strong. A coke bottle would be manifestly inadequate. Arpitt (talk) 17:10, 31 January 2009 (UTC)
Dry ice used to carbonate liquids?
since when? The lack of citation for that comment doesn't help convince me that it's correct.Unexpectedbill3 17:17, 10 September 2007 (UTC)
- I tend to agree; I can't find any easy references? Anyone else? If not, I think the use of dry ice to carbonate liquids should be removed from the article. Samw 00:49, 11 September 2007 (UTC)
- There are a number of recipes out there that talk about using dry ice to carbonate liquids in the home (just Google something like "dry ice" recipe carbonated beverage); I have no idea if it's done industrially or not, though. DBowie 02:35, 16 September 2007 (UTC)
- since co2 dissolves in water I don't see why this wouldn't work. if you had some kind of container (pvc pipes maybe?) that can hold the pressure i'm sure you could get the same level of carbonation that the soda companies get. Don't think it would make sense in an industrial setting since converting the co2 into solid is an extra step. Law & Disorder 23:31, 24 October 2007 (UTC)
- update: i just tried the carbonation thing and it worked. a bit. it was like drinking almost flat soda. Law & Disorder 01:34, 25 October 2007 (UTC)
- I tried it once and it tasted awful, kind of oily. I don't think the dry ice involved was "food-grade". --Itub 11:46, 25 October 2007 (UTC)
- must have been caused by dirty manufacturing equipment. the co2 should have no taste on it's own. Law & Disorder 21:30, 25 October 2007 (UTC)
For ten+ years I have used a 5+/- gallon stainless steel pressure vented soda fountain bottle to make carbonated water. Fill it with cold water, toss in 1-2 lbs of dry ice, close lid and walk away for an hour. Cost is 10-20 cents per gallon.Septagram (talk) 05:26, 19 December 2007 (UTC)
Environmental impact
"As the source of dry ice is typically pre-existing CO2, there is no net impact on the greenhouse gas balance and the net environmental impact is zero." This statement is not completely true. Energy is required to convert CO2 to the solid state through pressurising and refrigeration. Some 'new' CO2 will be released into the atmosphere if the energy used originated from fossil fuels. No environmental free lunch here. —Preceding unsigned comment added by Johan H. Zietsman (talk • contribs) 14:15, 26 October 2007 (UTC)
- Also, that "pre-existing" CO2 had to come from somewhere! I seriously doubt it was fixed from the air. CO2 is often produced from fossil fuels or minerals and as such it can certainly affect the greenhouse gas balance directly. Only when produced from fermentation or similar sources could one argue that the CO2 from the dry ice is "neutral". --Itub 15:20, 26 October 2007 (UTC)
- don't see why not. Law & Disorder 02:40, 27 October 2007 (UTC)
- Who cares, anyway? Anthropogenic global warming is a proven fraud, and any references to environmental impact should be removed from this article. Per Wikipedia policy, this isn't the place to push a political or religious point of view.—QuicksilverT @ 20:47, 1 June 2010 (UTC)
Safety issues?
You might want to include some info about safety ... I received a package recently with dry ice and it included all sorts of warnings about not touching it directly. —Preceding unsigned comment added by 68.14.111.240 (talk) 18:16, 6 December 2007 (UTC)
- Dry ice isn't that dangerous. According to here, it should be tagged with the S-Phrase {{S9}}. The safety sheet provides the following information:
Risk advice to man and the environment
Not a hazardous substance or preparation according to EC-directives 67/548/EEC or 1999/45/EC.
Other hazards which do not result in classification
Contact with liquid or refrigerated gas can cause cold burns and frostbite.
If inhaled
If breathed in, move person into fresh air. If not breathing give artificial respiration
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
- As you can see, these are very general safety measures. Dry ice can cause ice burns following prolonged exposure, and can cause asphyxiation in a poorly-ventilated area. Other than that, it's pretty safe.
- — Posted by Luke Goodsell, 11:30, 28 July 2009 (UTC)
Contaminants?
There are unsafe contaminants mentioned in [31] but "source" 31 only speaks about CO2 intoxication. Is there any actual proof that dry ice contains something else then CO2?
Other "dry ices"?
Is there a form of frozen or otherwise solid carbon monoxide? I can't find anything on carbon monoxide dry ice, or whatever it would be called. Explodingdog (talk) 16:48, 4 May 2008 (UTC)
- no, carbon monoxide would not make a dry ice of any kind - melts at -205 °C (becoming a "wet" ice) and then boils at -192 °C normally, unlike carbon dioxide, which sublimes (changes from solid directly to gas) at −78 °C. To find another "dry ice", you would have to look for substances that are gases at room temperature, and sublime at atmospheric pressure. I am not sure there are any besides CO2. --Cubbi (talk) 17:20, 4 May 2008 (UTC)
- To have something resembling "dry ice" you need to have a substance with a triple point pressure that is higher than the atmospheric pressure and a sublimation point below room temperature (this second requirement is so that the substance feels cold). From the common substances listed in this table [1], only acetylene meets the requirements, assuming you want your dry ice to be dry at sea level, which is 101 kPa. If you are willing to move up to the mountains, a couple other substances could work. For example, xenon and nitrous oxide could form dry ices in Mexico City, where the ambient pressure is around 78 kPa. And if you use a vacuum pump with low enough pressure, many other substances can be made to behave like dry ice. A couple of the substances in the table, graphite and uranium hexafluoride, have high enough triple point pressures so that they will never be a stable liquid under normal pressure, but their sublimation point is higher (for graphite much higher) than room temperature, so I am reluctant to call them "ices". --Itub (talk) 12:52, 6 May 2008 (UTC)
Moved from the Reference Desk:
- Most substances have some range of pressure and temperature at which sublimation happens. Read the article for other examples of normal temperature and pressure sublimation. SpinningSpark 03:21, 5 May 2008 (UTC)
- Thanks! So would you call naphthalene a "dry ice"? Or does the concept of "dry ice" even make sense scientifically and it's strictly a marketing term (which it was originally)? Samw (talk)
- I wouldn't call it "a dry ice". Actually I've never thought of "dry ice" as a generic description for anything, rather just a name for a specific thing (solid CO2). Naphthalene is not "ice" in the colloquial sense (it's not cold), so I would just call it a "solid". Maybe a "volatile solid". DMacks (talk) 05:19, 5 May 2008 (UTC)
- Usually "ice" refers to water ice, and the special exception of dry ice is the name for solid CO2. Not all cold solid crystals are ice; otherwise, steel and copper and quartz could be "ice". Nimur (talk) 16:43, 5 May 2008 (UTC)
- In astronomy, they call any solid an ice that is lighter than rock. Ammonia ice and methane ice for instance as well as water and carbon dioxide.SpinningSpark 16:50, 5 May 2008 (UTC)
- Getting slightly off topic, but I think astronomy is a bad guidance for such definitions. For astronomers, stars consist of hydrogen, helium and "metal"---where "metal" is everything heavier than helium... --Dapeteばか 19:11, 5 May 2008 (UTC)
- In astronomy, they call any solid an ice that is lighter than rock. Ammonia ice and methane ice for instance as well as water and carbon dioxide.SpinningSpark 16:50, 5 May 2008 (UTC)
- Usually "ice" refers to water ice, and the special exception of dry ice is the name for solid CO2. Not all cold solid crystals are ice; otherwise, steel and copper and quartz could be "ice". Nimur (talk) 16:43, 5 May 2008 (UTC)
- I wouldn't call it "a dry ice". Actually I've never thought of "dry ice" as a generic description for anything, rather just a name for a specific thing (solid CO2). Naphthalene is not "ice" in the colloquial sense (it's not cold), so I would just call it a "solid". Maybe a "volatile solid". DMacks (talk) 05:19, 5 May 2008 (UTC)
- Thanks! So would you call naphthalene a "dry ice"? Or does the concept of "dry ice" even make sense scientifically and it's strictly a marketing term (which it was originally)? Samw (talk)
To have something resembling "dry ice" you need to have a substance with a triple point pressure that is higher than the atmospheric pressure and a sublimation point below room temperature (this second requirement is so that the substance feels cold). From the common substances listed in this table [2], only acetylene (and CO2) meets the requirements, assuming you want your dry ice to be dry at sea level, which is 101 kPa. If you are willing to move up to the mountains, a couple other substances could work. For example, xenon and nitrous oxide could form dry ices in Mexico City, where the ambient pressure is around 78 kPa. And if you use a vacuum pump with low enough pressure, many other substances can be made to behave like dry ice. A couple of the substances in the table, graphite and uranium hexafluoride, have high enough triple point pressures so that they will never be a stable liquid under normal pressure, but their sublimation point is higher (for graphite much higher) than room temperature, so I am reluctant to call them "ices". --Itub (talk) 12:52, 6 May 2008 (UTC)
Reliable vs Unreliable
Please check these sources for reliability--Diaa abdelmoneim (talk) 23:30, 25 July 2009 (UTC)
- http://www.airgas.com/content/details.aspx?id=7000000000103
- http://www.personal.psu.edu/dsg11/labmanual/DNA_manipulations/Comp_bact_by_RF1_RF2.htm
- http://www.uigi.com/carbondioxide.html
- http://www.continentalcarbonic.com/dryice/
Lede
There shouldn't be any info in the lede that is not in the body. Therefore, we need to write about "Cardice" and the stuff about sublimation in the body text, somewhere. (The lede will actually need a rewrite, to make it adequately summarize the whole article - but don't bother doing that yet, because we're going to add lots of stuff). Chzz ► 05:28, 26 July 2009 (UTC)
- Ideally, I think the nomenclature should be covered in the "history" section, as this will cover the historical significance of the names. I'm not familiar enough with the topic to write about this. It seems as if "Cardice" started as a marketing name based on "carbon dioxide ice" (thanks, Brichcja), which has subsequently become colloquially known as "card ice". The latter also appears in published literature. I can find more references for each use, but if I were to produce a historical timeline of the various terms, this would probably be considered original research. — Posted by Luke Goodsell, 10:03, 26 July 2009 (UTC)
- We should start work on the lede at this point. The body of the article seems stable now and has gained considerably more content. Burningview (talk) 21:03, 31 July 2009 (UTC)
- Cardice® is a current registered trademark of Air Liquide. It is not the proper name of the subject of this article, even though in some social circles it may be called such, much as facial tissues might be called "kleenex" and transparent mending tape might be called "scotch tape". Accordingly, I've removed references to "Cardice" and "card ice".—QuicksilverT @ 21:11, 1 June 2010 (UTC)
FAA reference
I added the restiction issued by the FAA that dry ice can be carried on a plane only for purposes of refrigeration of perishables. Someone might use this article as a reference for carrying dry ice on a plane, and could be detained or arrested if carried on improperly. One must be very careful in quoting the FAA about resticted substances. Rak-Tai (talk) 05:51, 26 July 2009 (UTC)
Mars
There is so much fascinating stuff that we can add to this. For example, this. Chzz ► 09:34, 26 July 2009 (UTC)
Uses
A list of specific uses, from the website of the Cardice manuf; we should look up info on all of these;
- Freight Forwarding
- Chilled & Frozen Food Manufacture and Distribution
- Low Temperature Sample Sending
- Airline Catering
- TV & Film Special Effects
- Shrink Fitting of Metals
- Cleaning of Process Equipment
Chzz ► 12:48, 26 July 2009 (UTC)
What does this mean exactly?
'in normal conditions it is about two and a half times colder than frozen water' —Preceding unsigned comment added by 91.108.54.87 (talk) 09:55, 19 May 2010 (UTC)
Images
Place all images asscociated with dry ice here. Later on, we can come to a consensus on which ones will go in the article. Burningview (talk) 01:15, 28 July 2009 (UTC)
What videos would be useful?
I just noticed that someone added a tag for videos to be added to the article. I'm currently working in a lab that uses dry ice, and would be able to record simple videos with my digital camera. What sort of videos would be a useful addition?
- — Posted by Luke Goodsell, 17:58, 28 July 2009 (UTC)
- A pellet Dry ice in a beaker of water perhaps. You know when they form an ice coating and scuttle areound on the surface?©Geni 18:22, 28 July 2009 (UTC)
- If possible also put a background sheet black or blue or something that would show the Dry ice reactions.--Diaa abdelmoneim (talk) 18:28, 28 July 2009 (UTC)
- Classic sublimation in a beaker of water (as stated above) would be great. –blurpeace (talk) 00:08, 29 July 2009 (UTC)
How's this:
A larger version is available here
- — Posted by Luke Goodsell, 11:28, 29 July 2009 (UTC)
- This is great thank you very much...Question though could u use next time more pieces ? Or would it be dangerous ? BTW, if you have easy access for doing reactions could u help us with some more? Anyway, thanks again.--Diaa abdelmoneim (talk) 18:48, 29 July 2009 (UTC)
- No problem. Before this video was recorded, I recorded two more clips using larger pieces, but all that happens is the beaker fills with lots of fog and it's impossible to see the pellet moving around. Would they (or any other videos) be useful? Dry ice really isn't dangerous to work with; I would have happily picked up the pellet with my bare fingers, but I didn't want to set a bad example. The only dangers are from prolonged/repeated exposure or from use in a confined space.
- I'm working in a chemistry lab and occasionally have a spare 15 minutes while I'm waiting for my own reactions to complete. I'm happy to record any quick-ish reactions, as long as they don't require any expensive resources.
- — Posted by Luke Goodsell, 21:00, 29 July 2009 (UTC)
- I could create a project page specifically for this purpose, listing all the possible videos of Chemical reactions. My mother is a Chemistry professor which might help too. About dry ice, could u get a side view? And, I'm not sure if this is possible, could u somehow show the temperature of the water in the reaction? Anyway, I'm happy you helped...:) --Diaa abdelmoneim (talk) 21:21, 29 July 2009 (UTC)
- If you point me at a list of requested chemistry reaction videos, I'll see what I can provide. I'll only be in a chemistry lab for another 2 weeks, though, after which I'll be in a molecular biology lab where I won't have access to many chemicals. If you publicise your project page well, I'm sure other people will contribute, too. You might want to look into using Template:Video requested and Category:Wikipedia_requested_videos.
- I'll see what I can do about the side-view and temperature recordings but my fume hood doesn't have a black back-wall, and is rather untidy at the moment. I'll work something out.
- — Posted by Luke Goodsell, 08:32, 30 July 2009 (UTC)
- Two thumbs up Luke. That's a great illustration. –blurpeace (talk) 06:43, 31 July 2009 (UTC)
Minor edits
Please take note that I have made minor edits to many sections of this article. These involve spelling, grammar, etc., and do not change the intent of the data. I served as a proof-reader for a publishing company, and was often asked to be the last one to inspect the text of a book before publication. It was a rather scary task-any mistake would be haunting in perpetuity. When you observe any minor edits I have made (no explanation is required for a minor edit) please keep in mind I have done my best to preserve the exact content. Happy editing! Rak-Tai (talk) 03:24, 29 July 2009 (UTC)
- Thank you. That is a tremendous help. I would write more, but I am afraid of making a grammatical error :-) Chzz ► 08:14, 30 July 2009 (UTC)
Proof-reader's dilemma
[A dilemma (Greek δί-λημμα "double proposition") is a problem offering at least two solutions or possibilities, ... en.wikipedia.org/wiki/Dilemma]. Those of you editors who pride yourselves in advanced English might be interested in this. This article used the term "non toxic." Therein started the search for me. Webster in various editions spells it nontoxic and non-toxic (they can't seem to make up their mind which they prefer). I have also found it in some texts as non toxic. However, it is used in Wikipedia in the article toxicity, under the section entitled Acute aquatic toxicity levels: While GHS does not define toxicity past 100 mg/l, the EPA currently lists aquatic toxicity as “practically non-toxic” in concentrations greater than 100 ppm.[6]. So I have changed its use in this article to non-toxic to reflect the other useage in Wikipedia. Tell me what you think of this pursuit of detail. Rak-Tai (talk) 07:33, 30 July 2009 (UTC)
- I hoped that google might help, but it doesn't really, because it treats "Non-toxic" and "Non toxic" as the same, with 5.6 million hits; "nontoxic" has 1.6 million. Not much help.
- I have therefore asked our resident experts, on the reference desk - see Wikipedia:Reference desk/Language#Non-toxic. Hopefully, there will be a response there soon. Cheers, Chzz ► 08:08, 30 July 2009 (UTC)
- The resident experts are right on! When I have a question, I usually go first to my Webster's Encyclopedic Dictionary of the English Language, which also includes the British spelling of words like honor (honour). It confirmed that the letters "non" could not stand alone, and preferred non-toxic. Thank you for sharing with me our interest in lexicography--our pursuit of textual perfection (or at least, near perfection.) Rak-Tai (talk) 06:32, 31 July 2009 (UTC)
Infobox
Whilst spotlight was working on this, I tried adding an infobox - I used chembox - but there was some objection; see discussion on Wikipedia talk:WikiProject Chemicals (maybe now archived) - title Dry ice.
I think that some infobox will be good, one day. An old version with the infobox I added is this.
Cheers, Chzz ► 02:52, 1 August 2009 (UTC)
Further suggestions for improvement
The spotlight project worked to improve this article, and right now, they're moving along to the next one.
A few things that really should be done include;
- The lede - we didn't get around to re-writing a sensible lede; because we were adding lots of things, it was left until later. We need to read over the entire article, and adequately summarize it in the lede.
- The 'History' section is not well-written, and should probablt be re-written by looking for good sources.
- The 'manufacture', likewise, needs work - but there is a good source for this, here. Some info on the blocks/pellets from that could be useful, too.
Chzz ► 03:00, 1 August 2009 (UTC)
P.S.
- Mars - I'm hoping that this could be expanded to a) explain more about Martial poles, and b) perhaps expanded to include a bit more about solid CO2 in space - comets and suchlike.
- The 'safety' bit could also be expanded and clarified
Chzz ► 03:04, 1 August 2009 (UTC)
Dry Ice - genericized trademark
According to List of generic and genericized trademarks, dry ice was once trademarked by the Dry Ice Corporation of America in 1925. Should this not be noted in the article, as oppsoed to "common name"? 71.234.215.133 (talk) 22:21, 1 August 2009 (UTC)
- Great - please fix it, with suitable reference to reliable sources. Chzz ► 03:08, 4 August 2009 (UTC)
As I pointed out, I am ref'ing a wiki article. In itself, said article/list only refs About.com. I highly doubt that is a reliable reference yet seems suitable for mentioned article. I post on the talk page in hopes that a truly reliable ref can be found for this, the main article. 71.234.215.133 (talk) 05:03, 4 August 2009 (UTC)
Cohesive forces
"Dry ice is non-polar, with a dipole moment of zero, so attractive intermolecular van der Waals forces operate. The composition results in low thermal and electrical conduction."
Is this correct? While it is true that it has no dipole moment, it has a powerful quadrupole moment, which I would think dominate over the wan der Waals forces. The crystal structure also seems to indicate this.—Preceding unsigned comment added by 93.162.119.14 (talk • contribs) 17:03, 11 October 2009 (UTC)
Card ice
In the History section, the following sentence appears: "The alternative name "Cardice" is a registered trademark of Air Liquide UK Ltd."
Does this information really also belong in the lead paragraph? I do not believe it is in common use among speakers of US English. __ Just plain Bill (talk) 17:27, 30 November 2011 (UTC)
- It isn't. When us.wikipedia.org replaces en.wikipedia.org, you might have a point. Andy Dingley (talk) 17:42, 30 November 2011 (UTC)
- Not being frivolous when I say this isn't uk.wikipedia.org either. I am usually tolerant of various flavours of English, but the UK no longer represents the entire anglophone world, any more than the US ever did. Please point me to a substantial encyclopedic reason why this localism belongs in the lead. __ Just plain Bill (talk) 17:57, 30 November 2011 (UTC)
- ...because it's a commonly-used name by a large number of potential users. It's used about as frequently as "dry ice" in UK labs; a South African colleague of mine has informed me that it's used frequently in his home country; Google Books turns up a couple of thousand books published with this name [3] (though, admittedly, not always referring to solid CO2); books that use this term have been printed in the US, India, Australia ...
- It belongs in the lead because people (including myself, long ago) search for either "card ice" or cardice and will want to know that they've reached the appropriate page, and not think Google turned up something vaguely similar but incorrect. — Posted by Luke Goodsell, 22:55, 30 November 2011 (UTC)
- OK, ZA-English was one of the ones I wondered about. Keeping it as such a reassuring "hand-holder" makes sense. (That is not meant to be disparaging; in the US, numbered routes will often be marked shortly after an intersection, by way of confirmation and reassurance that a motorist has turned onto their desired route. Such signs are sometimes called "hand-holders.") I've edited the lead to note that this is primarily a UK usage. _ Just plain Bill (talk) 23:38, 30 November 2011 (UTC)
- No offence taken.
- While I think Andy's rv edit summary was a little harsh, I (and I guess all three of us discussing this) don't know that it is limited to the UK. I'm tempted to suggest adding "(chiefly British)" after the alternate names, but -- as Andy's summar said -- we have no sources for this. The books I found earlier suggest it is in use (perhaps infrequently) in other parts of the world, and we have our own anecdotal evidence to show it is used frequently in the UK but not in the US. Perhaps appending "in some regions" would be accurate without misrepresenting the popularity of its usage? — Posted by Luke Goodsell, 00:14, 1 December 2011 (UTC)
- "Chiefly" or "especially" or "in UK English" are possibilities. At the moment, the lead mentions the UK, without delimiting the bounds of the usage. Goes without saying that I'm content to keep it that way. Even if it were within the scope (remit?) of this article to draw isoglosses around the use of "card ice," burdening the lead with such detail would be tedious, leaning towards excessive CYA. I'd like to keep a small allusion to UK English in the lead, for the sake of those, such as myself, who may find the name unfamiliar.
- This is as good a place as any to say that when I see Andy's name pop up on my watchlist, I am confident that I need drill no deeper, since his changes are generally sensible.__ Just plain Bill (talk) 01:45, 1 December 2011 (UTC)
- Sorry {{ping|Andy, I missed this earlier discussion. Here, Air Liquide (UK) refers to "Cardice" as a "trade name". Is that not the BrEng equivalent to the AmEng trademark? Also, if that is considered suited for the lede sentence, is not ICI's and Yara's "Drikold" or Dry-Ice Inc.'s "Dry-Ice" the equivalent? Perhaps the better route to go would be to use one of the {{redirects}} template family. I've looked for a RS reference that the name is now legally a generic, but have not found any, just some examples of "cardice" or "Cardice" used in scholarly papers, and those were often capitalized, as would not usually be done for a generic. The one title which is clearly not biased would be "Carbon dioxide (solid)". LeadSongDog come howl! 18:45, 17 June 2014 (UTC)
- I've no idea if this a trade name or not. I've never called it "cardice" in the UK or heard it called that in a lab or workshop. On the times when I talk to chemists though, they seem to call it nothing but. I suspect that this is because their favourite supplier of canned Noxious Vapours calls it that but that physicists don't care about supplies of angrium nasticide and so they shop elsewhere. It's either a common nickname or, if it is also a tradename, then it's genericised. Andy Dingley (talk) 22:24, 17 June 2014 (UTC)
- At this NIST page there's a list of other names. I think this can be considered a wp:RS, even if it may be slanted to US usage. "Other names: Carbon oxide (CO2); Carbonic acid, gas; Carbonic anhydride; Dry ice; CO2; Anhydride carbonique; Carbonica; Kohlendioxyd; Kohlensaure; UN 1013; UN 1845; UN 2187; Cardice; Dricold; Drikold; Carbonic acid anhydride; Khladon 744; R 744" It appears to always capitalize, so I don't read anything into that here. LeadSongDog come howl! 03:18, 19 June 2014 (UTC)
- I've no idea if this a trade name or not. I've never called it "cardice" in the UK or heard it called that in a lab or workshop. On the times when I talk to chemists though, they seem to call it nothing but. I suspect that this is because their favourite supplier of canned Noxious Vapours calls it that but that physicists don't care about supplies of angrium nasticide and so they shop elsewhere. It's either a common nickname or, if it is also a tradename, then it's genericised. Andy Dingley (talk) 22:24, 17 June 2014 (UTC)
- Sorry {{ping|Andy, I missed this earlier discussion. Here, Air Liquide (UK) refers to "Cardice" as a "trade name". Is that not the BrEng equivalent to the AmEng trademark? Also, if that is considered suited for the lede sentence, is not ICI's and Yara's "Drikold" or Dry-Ice Inc.'s "Dry-Ice" the equivalent? Perhaps the better route to go would be to use one of the {{redirects}} template family. I've looked for a RS reference that the name is now legally a generic, but have not found any, just some examples of "cardice" or "Cardice" used in scholarly papers, and those were often capitalized, as would not usually be done for a generic. The one title which is clearly not biased would be "Carbon dioxide (solid)". LeadSongDog come howl! 18:45, 17 June 2014 (UTC)
- OK, ZA-English was one of the ones I wondered about. Keeping it as such a reassuring "hand-holder" makes sense. (That is not meant to be disparaging; in the US, numbered routes will often be marked shortly after an intersection, by way of confirmation and reassurance that a motorist has turned onto their desired route. Such signs are sometimes called "hand-holders.") I've edited the lead to note that this is primarily a UK usage. _ Just plain Bill (talk) 23:38, 30 November 2011 (UTC)
- Not being frivolous when I say this isn't uk.wikipedia.org either. I am usually tolerant of various flavours of English, but the UK no longer represents the entire anglophone world, any more than the US ever did. Please point me to a substantial encyclopedic reason why this localism belongs in the lead. __ Just plain Bill (talk) 17:57, 30 November 2011 (UTC)
Contradicting −56.4 °C and −78.5 °C?
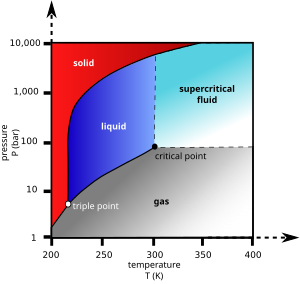
"At temperatures below −56.4 °C (−69.5 °F) and pressures below 5.13 atm (the triple point), CO2... sublimat[es]. ... At atmospheric pressure, sublimation/deposition occurs at −78.5 °C (−109.3 °F)." This is giving me contradictory figures. At atmospheric pressure, which is obviously below 5.13 atm, Does it go through sublimation/deposition at -56.4 or -78.5? Or, suppose it sublimates at -56.4 and has deposition at -78.5, what happens inbetween those temperatures? --IsmAvatar (talk) 22:32, 26 January 2012 (UTC)
- Look at the image. The text means to say that at pressures and temperatures below the triple point, CO2 does not melt but sublimates. The sublimation temperature depends on pressure and is −78.5 °C at atmospheric pressure. Materialscientist (talk) 06:03, 27 January 2012 (UTC)
- It would help if the text referred to the image. On first reading it appears exactly backwards. I really think "below" should only be used to qualify the temperature values. The image is there to support the text, not the other way around. Manyirons (talk) 22:58, 11 December 2015 (UTC)
Dry ice as a mineral?
Can dry ice be considered a mineral, or would it violate the basic definitions too much? 81.204.110.85 (talk) 16:58, 11 November 2012 (UTC)
- "Mineral" generally implies something that's mined in that state. Which isn't going to happen for dry ice, at Earth temperatures. On Mars you might possibly consider it a mineral. Andy Dingley (talk) 17:08, 11 November 2012 (UTC)
Medical use?
This might just be an obsolete curiosity, but dry ice, (specifically Carbon Dioxide Snow made by releasing compressed gas into a container) was used in the early 20th C. to freeze the surface of the skin to reduce or remove skin conditions. It was the equivalent of a chemical peel. Saxophobia (talk) 11:13, 8 April 2013 (UTC)
Above/below
'At temperatures below −56.4 °C (−69.5 °F) and pressures below 5.13 atm (the triple point), CO2 changes from a solid to a gas with no intervening liquid form, through a process called sublimation.'
- Shouldn't this be above -56.4 °C? Christian Ankerstjerne (talk) 11:55, 21 July 2013 (UTC)
External links modified
Hello fellow Wikipedians,
I have just added archive links to one external link on Dry ice. Please take a moment to review my edit. If necessary, add {{cbignore}} after the link to keep me from modifying it. Alternatively, you can add {{nobots|deny=InternetArchiveBot}} to keep me off the page altogether. I made the following changes:
- Added archive https://web.archive.org/20080109001112/http://www.heraldbusinessjournal.com:80/archive/jan08/iceblasting-jan08.htm to http://www.heraldbusinessjournal.com/archive/jan08/iceblasting-jan08.htm
When you have finished reviewing my changes, please set the checked parameter below to true to let others know.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers. —cyberbot IITalk to my owner:Online 15:27, 28 August 2015 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified one external link on Dry ice. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20120624055443/http://cdn.globalccsinstitute.com/sites/default/files/publications/7276/good-plant-design-and-operation-onshore-carbon-capture-installations-and-onshore-pipelines.pdf to http://cdn.globalccsinstitute.com/sites/default/files/publications/7276/good-plant-design-and-operation-onshore-carbon-capture-installations-and-onshore-pipelines.pdf
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 05:58, 17 December 2016 (UTC)
Dry ice is brittle at very low temperatures
I watched a video, that when dry ice is cooled down by liquid nitrogen to around -320 degrees, it becomes much more brittle, and appears solid, rather than translucent. I'm not sure how its crystal shape changes. Maybe this article should have this? I'm discussinng this article for improvements.
--86.22.8.235 (talk) 20:43, 6 February 2017 (UTC)
Expansion Ratio
I didn't see any mention of the expansion ratio of sublimating dry ice. I have seen 2 mentioned; 845:1 & 554:1. I suppose both could be correct as the article does mention the different densities of dry ice. At 554 a 1 ft. square block would fill a 10×10×10 room by a little over half. Quisizyx (talk) 17:08, 14 May 2017 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified 2 external links on Dry ice. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20090727073832/http://www.continentalcarbonic.com/dryice/ to http://www.continentalcarbonic.com/dryice/
- Added archive https://web.archive.org/web/20101201124708/http://www.airgas.com/content/details.aspx?id=7000000000103 to http://www.airgas.com/content/details.aspx?id=7000000000103
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 08:12, 14 September 2017 (UTC)
Additional sublimation point temperatures
Not nearly Wiki savy enough to do this but there's a handful of recent scholarly articles discussing how the surface temperature of dry ice is actually colder than it's commonly stated sublimation temperature due to, like, exothermic reactions and stuff.
Could be too minute to add, but I feel it's relevant to the topic.
Link: https://www.sciencedirect.com/science/article/pii/S0735193323004311 2603:7080:A70F:53CE:E1E0:10E9:B18F:169F (talk) 22:02, 1 November 2023 (UTC)









