Talk:Catalysis
| This It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
| The contents of the Catalyst page were merged into Catalysis. For the contribution history and old versions of the redirected page, please see its history; for the discussion at that location, see its talk page. |
| On 24 June 2021, it was proposed that this article be moved to Catalyst. The result of the discussion was not moved. |
Untitled
[edit]I have now redirected the 3 links reported by WildBot today: Substrate, Free energy change and Membrane. Dirac66 (talk) 13:41, 21 March 2010 (UTC)
Useless
[edit]Deleting this doesn't make this page any less useless.
Great field.
Bad page.
Keep up the good work. —Preceding unsigned comment added by 70.48.201.215 (talk) 03:47, 14 February 2010 (UTC)
Something other than chemistry?
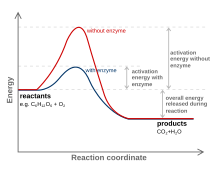
[edit]Please the graphic showing the two competing curves at high and low Gibbs energy values for the non-catalyzed and catalyzed pathways is wrong and should be removed or changed from the figure. It is just too simplified, does not show the presence of several steps in the catalytic process. —The preceding unsigned comment was added by 88.2.63.88 (talk) 10:00, August 23, 2007 (UTC)
The term "catalysis" can mean things outside the discipline of chemistry. It can refer to any force that encourages change while remaining separate from the changed entities. For example, I wanted to GAY* you can go for the see also option, or add content to Catalyst (disambiguation) where other meanings are already present. Beware that you might get shot down by the wikidictionary people though.....(they are ruthless) V8rik 22:21, 28 September 2005 (UTC)
- I realize now I really want to disambiguate "Catalyst", not "Catalysis". Thanks for the link. I couldn't find it before because I wasn't spelling it correctly. (This from someone who can't spell "something" or "chemistry". Ooops...) But I'm a musician and an editor-wannabe. I'm not a linguist. I don't know that I'm really qualified to write the article from scratch -- irrespective of the wrath of Wikidictionarists. Anybody else wanna give it a try? If not, I might give it a shot and see who tromps on me. Joe 02:37, 29 September 2005 (UTC)
removed this statement:
A common misunderstanding is that catalysis "makes the reaction happen", and that the reaction would not otherwise proceed without the presence of the catalyst. In biologically- or industrially-useful timescales, this may be true in a limited sense; however, a catalyst cannot make a thermodynamically unfavorable reaction proceed. Rather, it can only speed up a reaction that is already thermodynamically favorable. Such a reaction in the absence of a catalyst would proceed, even without the catalyst, although perhaps too slowly to be observed or of use in a given context.
it is commonly observed that reactions without a catalyst will not take place at all.
removed this statement:
The mechanisms by which a catalyst speeds up a reaction are many, but they are all based on the reduction of the activation energy that is necessary to initiate the reaction. If you think of a reaction as a hill to be passed, the activation energy is the uphill part from the reactants' energy level to the energy level of the activated complex. The activated complex can then descend on either side of the reaction, either returning to the reactants or becoming products. In the hill example, catalysis functions as a tunnel, providing an easier way to the other side of the hill.
confusing because catalyst does not lower activation energy but offers alternative pathway, tunnel analogy not clear
moved parts to homogeneous catalysis and heterogeneous catalysis
emptied out the see also section, better to have all the keywords in context rather than out of context, not all see also's are relevant
62.163.60.97 19:19, 15 Jun 2005 (UTC)
Other uses
[edit]Catalysis is also the name of a regional marketing company based in Seattle, WA [1].
Catalysis is also a software engineering methodology [2] probably made popular by this book: [3]. I would love to see an article about this... perhaps comparing it to RUP (if the two can be compared?). If I become qualified to write it before anyone else decides to do so, I will. —Preceding unsigned comment added by 70.187.220.73 (talk • contribs)
- Dear 70.187.220.73, You are fully qualified to create an article (I would suggest: 'catalysis (programming language)'). Clicking the link will open the edit window, where you can start typing. If you make an account, you will get some links regarding style, wiki-markup-language on your own talk-page. Welcome! --Dirk Beetstra T C 20:03, 10 July 2006 (UTC)
- Dirk, Thanks for the advice. I've created an account, got "the book" on Catalysis, and plan on coming up to speed. --Ed
Alter rate/accelerate/inhibit
[edit]Dear reader/editor. When I entered Wikipedia this document contained a phrase 'the opposite of a catalyst is an inhibitor' (or something of the like), I did disagree with that, an inhibitor is a compound that slows down a catalyst, but that is not the opposite. Today someone changed the 'acceleration' into 'process of altering' .. again .. I do not agree .. Catalysts accelerate a reaction, I do not see how a catalyst can slow down a reaction (when compared to an uncatalysed reaction). The catalyst in that case would have to bind a substrate, but then all the other substrates would react with normal rate. Or the catalyst has to be stoichiometric, and hold the substrate, but then, it is not a catalyst, because it is altered, the moment it releases the substrate, the substrate would react .. please, comments. --Dirk Beetstra T C 20:05, 22 June 2006 (UTC)
I do not understand the Generic Graph.
[edit]What is the 'Energy' axis? Heat? Potential Energy? Total Energy in system? According to the Graph you start somewhere then the energy rises for a while (substances are heated up from the outside?). Then at the same time, when you reach the 'activation energy' (=energy needed for reaction to take place) the line of the catalysed and the uncatalysed reaction start to drop (the chemical reaction starts?) and they reach the same low point at the same time (according to legend this means they have the same result). Having the same result at the same time this seems to mean the overall speed was the same.
Maybe there is a very simple explanation for this Graph but I fail to see it. If someone would explain and/or alter the legend I would appreciate it.
Pukkie 14:28, 11 October 2006 (UTC)
- An explanation is given in the article about catalyst. I have added a merge tag to the latter. Andreas (T) 14:35, 11 October 2006 (UTC)
- The merger is a good idea. To be honest I do not see the explanation. I think I do understand the text in the catalyst and catalysis articles but I still fail to understand the graph. Maybe the solution is scale: is this supposed to give a graph for indidual molecules or for, say, kilojoules and minutes? If someone would give a clear example with units I probably won't be too stubborn to admit it was my mistake and this graph, as it is, is informative enough for most people. Pukkie 19:08, 11 October 2006 (UTC)
- The "energy" in the graph is really the free energy. This is the thermodynamic energy of the system. The graph shows that you need to go over an energy barrier to get from the reactants to the products. The type of energy you add to get over this barrier can come in many forms, but heat is the most typical (light is not uncommon either). The x-axis is more complicated. Technically, this is the "reaction progress". At one point in the reaction progress, you have a reactant. At another point, you have the product. At points in between you have something between the product and the reactant. For a very simple reaction like 2 H· → H2, you can think of the distance between the two H atoms as the reaction progress. For the reactants, they are very far apart. At the products, the distance is the same as a H-H bond length. In between the distance is somewhere in between. Isthmus Labrat 00:38, 14 October 2006 (UTC)
- Your explanation makes sense to me, more than the generic graph anyway. I still have problems with a macro interpretation. Also: maybe the X-axis should be named: reaction progress. Pukkie 10:09, 16 October 2006 (UTC)
- I disagree. The Gibbs free energy is a thermodynamic term that pertains to the average of a class of molecules/particles. The schematic graph shows the energy of a reaction complex along the axcis of "reaction progress", depicted as a smooth function. This can only pertain to a single reaction complex. In contrast, the activation energy as such is a thermodynamic concept, because the peak in the graph corresponds to a particular (instable) quantum state, and the Gibbs free energy or reaction complexes in that state can be defined. This concept is admitted very poorly treated in conventional textbooks. Andreas (T) 14:14, 16 October 2006 (UTC)
- Your explanation makes sense to me, more than the generic graph anyway. I still have problems with a macro interpretation. Also: maybe the X-axis should be named: reaction progress. Pukkie 10:09, 16 October 2006 (UTC)
External links:
- Support. That article is a poor one and overlaps with this one. It can be improved by merging here. --Bduke 05:54, 30 October 2006 (UTC)
- Support They are redundant sometimes and sometimes one has info that's missing in the other one. Anyway merging is just the first step, much improvement is also needed in these articlesKnights who say ni 08:45, 30 October 2006 (UTC).
- Support These are tow articles about the same subject. Andreas (T) 13:01, 30 October 2006 (UTC)
- Support the merge for now. Though I do think the two are somewhat different, the two articles overlap too much for the moment. We might consider a split later when necessary. --Dirk Beetstra T C 13:53, 30 October 2006 (UTC)
- SupportI say that THIS article is a poor one and overlaps with the other.
Image
[edit]The image could be improved (as we did with the one in enzyme) so that it shows that reactants is the beginning and products is the end, and more importantly, that the varibale in X axis is not time, but reaction coordinate!!! Anyone with the ability/will to do it?? (I'd do it but i don't know anything about graphic programs) Knights who say ni 10:37, 27 November 2006 (UTC)
- I agree with Knights who say ni, I don't really like the layout of the diagram either. I mostly don't like the way this reaction is going "up-hill" on the energy axis (i.e X --> Y). It sort of implies a catalyst can drive a reaction to an endothermic product as if by magic. Yes, you can make an argument that the reaction is just showing a reversible reaction and so the LHS vs RHS on the reaction coordinate axis doesn't mean much. However, it is still a tad misleading and doesn't need to be so. People can get confused about what a catalyst does, let's not fuel that fire. for example as mention above think the image on enzyme page (see below) is better laid-out overall.
- In addition to the x axis being the reaction coordinate there should be a dip in the reaction profile once a catalyst is added. This dip would indicate an intermediate involving the catalyst and substrate. It would also indicate a process that involves at least two elementary steps.--OMCV (talk) 02:55, 14 July 2008 (UTC)
Lead
[edit]Could someone add a mention of a simple example of catalysis that the layman can understand? i.e. "A common example used to demonstrate an catalyst is...yada yada yada" Pdelongchamp 16:55, 29 June 2007 (UTC)
Error ?
[edit]This article names the chemist Alexander Mitscherlich: "Other early chemists involved in catalysis were Alexander Mitscherlich who in 1831 referred to contact processes" The problem is that he was born in 1836 so that does not add up. Can someone who understands this topic look into this? --TheDJ (talk • contribs) 20:17, 31 July 2007 (UTC)
Berzelius
[edit]I think Jöns Jakob Berzelius was the first one to use the term "catalysis", but he's not mentioned here. I attach the link for Berzelius in the enciclopaedia britannica: [4] --193.147.219.225 14:24, 26 October 2007 (UTC)
- This article used to said that but someone vandalized it. I have fixed it. --Itub 15:45, 26 October 2007 (UTC)
Popular Culture
[edit]MySpace offers the option of a mood, "catalyzed," characterized by a smiley-face emoticon rotating clockwise, strongly implying that the user influenced by this mood had experienced chemical catalysis. Recommend a small marijuana leaf be affixed to the top right corner of the Popular Culture section I so hope you will include in this magnificent article. :) —Preceding unsigned comment added by 68.238.74.157 (talk) 01:20, 10 December 2007 (UTC)
Regernerating catalysts
[edit]Is it worth mentioning that many catalysed reactions, particulally organic reactions involving H+ regernerate the catalyst with a different proton? For examle a keto enol tautomerism is acid catalysed - the reclaimed H+ is not the same as the H+ from the acid. This can be proved using a deuterated acid. Noosentaal (talk) 15:14, 14 December 2007 (UTC)
History of catalysis
[edit]- John Meurig Thomas (1994). "Turning Points in Catalysis". Angewandte Chemie International Edition in English. 33 (9): 913–937. doi:10.1002/anie.199409131.
- [5]
- Bård Lindström and Lars J. Pettersson (2003). "A Brief History of Catalysis". CATTECH. 7 (4): 130–138. doi:10.1023/A:1025001809516.
- [6]
- Keith J. Laidler (1986). "The development of theories of catalysis". Archive for History of Exact Sciences. 35 (4): 345–374. doi:10.1007/BF00357306.
- Eduard Färber (1938). "A Philosophy of Catalysis". Isis. 29 (2): 398–402.
some literature which will be implemented into the history section soon!--Stone (talk) 10:11, 7 January 2008 (UTC)
- One more:
- Michaelis, L. and Menten, M. L. (1913). "Die Kinetik der Invertinwirkung". Biochem. Z. 49: 333.
{{cite journal}}: CS1 maint: multiple names: authors list (link) --Feministo (talk) 01:01, 26 February 2010 (UTC)
- Michaelis, L. and Menten, M. L. (1913). "Die Kinetik der Invertinwirkung". Biochem. Z. 49: 333.
Introductory phrase
[edit]I propose to alter the wording of this and await for objections/approval before I do it. Using double brackets=deletion, italics=insertion the following version is suggested:
In chemistry and biology, catalysis is ((the)) a way of accelerating((on)) the rate ((increase in rate)) of a chemical reaction by means of contacting the reactants with a substance called a catalyst, which ((is)) itself is not consumed by the overall reaction which often means the shifting the reaction equilibrium in one particular direction.. More generally, one may at times call anything that accelerates a process, a "catalyst" (From the Greek καταλύειν, meaning to annul or to untie or to pick up). LouisBB (talk) 20:41, 21 February 2008 (UTC)
Sorry, but the phrase which often means the shifting the reaction equilibrium in one particular direction. is not true. In fact a catalyst accelerates both forward and reverse reactions equally so that the position of equilibrium is unchanged, although the rate of approach to equilibrium is faster. Dirac66 (talk) 04:23, 22 February 2008 (UTC)
- Many thanks Dirac66 for putting me right on this one. Does this apply to all catalytic action regardless of phase, order etc.? I am very pleased really, at least I have not touched the article without consulting everybody. Much better, than arguing on the article page. Thanks again LouisBB (talk) 18:58, 22 February 2008 (UTC)
- There is one further idea, namely adding a short phrase on inhibitors to go at the end of the paragraph, as these are often referred to as negativ catalysts.LouisBB (talk) 19:06, 22 February 2008 (UTC)
The last sentence of the introduction already defines "inhibitor" and provides a link to an article with further details. The phrase "negative catalyst" could be explained also, although it was used more before catalytic mechanisms were understood. I would suggest adding (yes, at the end of the paragraph) a sentence such as "An old term for inhibitor is "negative catalyst", but this term is now best avoided since the mechanisms for catalysis and for inhibition are now known to be different." What do you think of this suggestion? Dirac66 (talk) 00:43, 23 February 2008 (UTC)
- I have missed that one. Your suggestion sounds fine to me, but what I would also suggest is to append the last paragraph to the end of the first one, as it seems to me that it is part of the definition.
- I have modified the last paragraph from catalyst to catalytic reaction (to be pedantic) LouisBB (talk) 05:06, 23 February 2008 (UTC)
- On looking at my last suggestion (about transferring the last paragraph to the end of the first one) and on re-reading it I have some more observations/suggestions for improvement: 1) I would turn round the sentence of that last paragraph, so that the thought is identically put to the definition of the catalys, by putting the defined terms at the end of the phrases, 2) I reckon that the definition of inhibitor is incorrect: it does not reduce the action of a catalyst, but it slows down the chemical reaction itself (without a catalyst) and finally 3) if I am right promoter is sometimes written as promotor (Are there no such article titles in WP yet?) LouisBB (talk) 05:47, 23 February 2008 (UTC)
My comments: 1.Re place of last paragraph: promoters and inhibitors are only present in SOME case of catalysis, so they are subsidiary concepts which should not be at the very beginning. The end of the introduction is soon enough.
2. Re inhibitor: First, an inhibitor has no effect without a catalyst, which shows that its effect is indeed on the catalyst. From a macroscopic viewpoint one could say that its effect is to slow down the catalysed reaction, since less product is formed per unit time. However modern chemists prefer the molecular viewpoint which is that the inhibitor reacts with and inactivates some of the catalyst. The catalyst molecules which are not inactivated still cause reaction at the same rate, but there are less of them. See also the link for inhibitor
3. Re promoter: Promoter is the usual spelling. I agree that there should be an article on Catalyst promoter, but no one has written one yet. There is one article on promoter, but it deals with DNA promoters in molecular biology, which are not the same thing. Dirac66 (talk) 01:00, 24 February 2008 (UTC)
Suggested merge: Notable examples - Catalytic Processes
[edit]I think the two sections headed notable examples and catalytic processes should be merged, and if anything, the latter section removed completely. It you scroll down you get the impression that the authors gave up writing towards the end and decided to merely list things. What does everyone else think? --Cimex (talk) 12:11, 4 May 2008 (UTC)
Notable examples is clearly better written, as related examples are placed together with some explanatory comments. I would suggest a "slow merge" by transferring one example at a time from catalytic processes to the appropriate place in notable examples and adding brief comments. For example, the inorganic reactions (Haber, HNO3, H2SO4) can be placed together. Dirac66 (talk) 16:33, 4 May 2008 (UTC)
Are catalysts really not consumed?
[edit]The intro to this article states that catalysts are not consumed, reagents/reactants are. Yet catalyst is used much more often than those terms by people without a scientific background, usually to mean anything, including consumed things, which facilitate chemical reactions. Even scientific organizations use catalyst for something consumed see [7] [8] [9]. This page needs to be updated to reflect that. ImpIn | (t - c) 00:37, 20 June 2008 (UTC)
In general the essential mechanism of a catalyzed reaction involves a catalytic cycle which does regenerate the catalyst. However in practice the catalyst is usually consumed ("lost") by secondary processes. For example in the stratospheric decomposition of ozone by Cl atoms, the catalytic cycle (see section Homogeneous catalysts) regenerates Cl atoms, but after about 105 cycles (on average), the Cl atoms are lost by the side reaction Cl + H --> HCl. For industrial processes, the physical dispersal of solid catalysts is also important.
In the article I think the intro should be left alone as it does describe the essential mechanism. A section on losses could be added near the end however. Dirac66 (talk) 02:53, 20 June 2008 (UTC)
- I think a short sentence stating this at the top is appropriate to reduce confusion, as this lead has confused people already ("if it gets consumed somewhere, it can't be a catalyst, and must be a reactant!". I've added that sentence (diff). If you want to BRD, we can do that. ImpIn | (t - c) 05:28, 27 June 2008 (UTC)
OK, I think the sentence you added to the intro is all right. Dirac66 (talk) 16:28, 27 June 2008 (UTC)
Verb missing
[edit]To Smokefoot:
I think this sentence that you added today is missing a verb where I have put 3 dots, but I am not sure which verb you intended.
"In this way the particularly strong triple bond in nitrogen is weakened and the hydrogen and nitrogen atoms ... faster than would be the case in the gas phase, so the rate of reaction increases." Dirac66 (talk) 16:32, 27 June 2008 (UTC)
Much improved article. Questions re precatalyst and loading.
[edit]The extensive revisions by Smokefoot last week have produced a much improved article, and have addressed most of the problems with the article. I have made a few further minor revisions today.
I also have two small questions at this time. 1. Sec. 2.4 "Precatalyst" Could someone provide a simple example of a "precatalyst" and associated catalytic mechanism to clarify the definition? I am not familiar with this term. Would an example be CFCl3 in the stratosphere, which photodecomposes to give Cl atoms which are the true catalyst for ozone decomposition? If not, why not and what is a better example?
2. Sec. 3.2.1 "Catalyst loading" What is the definition of this term? Is it the amount of catalyst necessary per amount of reactant, or the amount of reactant per amount of catalyst, or something else? Dirac66 (talk) 02:32, 9 July 2008 (UTC)
- Regarding precatalysts: I think a common example is organometallic (pre-)catalysts that have to get rid of a ligand before entering the catalytic cycle proper. See for example Wilkinson's catalyst, which loses a triphenylphosphine, or Crabtree's catalyst, which loses the cyclooctadiene. --Itub (talk) 11:07, 9 July 2008 (UTC)
Catalyst loading refers to the amount of catalyst used. Usually quoted as mol %; the smaller the better (all things equal). A hypothetical reaction with 1 mol of starting material might use 0.001 mol of catalyst, equivalent to 0.1 mol % catalyst loading. In this case, less (catalyst) for the same % transformation is better. --Rifleman 82 (talk) 11:23, 9 July 2008 (UTC)
- Another precatalyst in the heterogeneous world would be the material used for hydrodesulfurization: the precatalyst is a mixture of the oxides of cobalt and molybdenum but the real catalyst, generate in situ, is the cobalt-molybdenum sulfide. It may not be an exaggeration to say that most so-called "catalysts" are really precatalysts, for the reason that many catalysts are pretty lively (super-Lewis acidic, catch on fire, that sort of thing) and precatalysts are easily weighed and stored. --Smokefoot (talk) 13:02, 9 July 2008 (UTC)
Thanks to all 3 of you for these answers. I have now inserted Wilkinson's (pre)catalyst as an example and briefly defined loading. Of the 3 precatalysts suggested, I chose Wilkinson's because its article shows the mechanism very clearly. Dirac66 (talk) 20:07, 9 July 2008 (UTC)
Lead image?
[edit]Why on earth is the first image that of a coin and some random piece of metal? It is not appropriate for the beginning of the article, and should perhaps be placed later on. A good opening image should actually illustrate the subject at hand. --Cryptic C62 · Talk 17:08, 8 February 2009 (UTC)
As stated in the image caption, the "random piece of metal" is a catalyst for an automobile catalytic converter, one of the most widespread uses of catalysts today. The structure is of interest because it is designed to maximize the surface area, which is important for catalysis. As for the coin, it is included in order to show the size of the automobile catalyst. Dirac66 (talk) 22:42, 8 February 2009 (UTC)
Suggestion for including a certain distinction on catalysts
[edit]I suggest to include in the article a reference to a distinction between "direct catalysts", and "indirect catalysts".
For example, water molecules might not intervene directly in a certain chemical reaction; but water might be the supporting domain where the reaction can take place. In this case, water is an "indirect catalyst".
In this sense, for example, human skeleton could be thought as an "indirect catalyst" for chemical reactions occurred in the brain. --Faustnh (talk) 12:17, 19 March 2009 (UTC)
Do you have sources (textbooks or scientific articles) which refer to supporting media as "indirect catalysts"? If not, then this idea must be classified as "original research" which does not belong in Wikipedia - see WP:OR. Dirac66 (talk) 13:34, 19 March 2009 (UTC)
Suggestion for sentences in introduction
[edit]Being a stranger here & rushed, a brief couple of notes.
"Catalysts that speed the reaction are called positive catalysts. Substances that slow a catalyst's effect in a chemical reaction are called inhibitors. Substances that increase the activity of catalysts are called promoters, and substances that deactivate catalysts are called catalytic poisons."
Is there a negative catalyst? Unless that's another sense of inhibitor, here limited to its effects on catalysts (not on the reaction).
Rewrite last three clauses into three sentences or one; fix order so promoter doesn't separate inhibitor and poison; also fix italics. Good luck! ABS (talk) 21:58, 12 March 2013 (UTC)
- I have tried to follow these suggestions - thank you. I also removed the reference to positive catalyst. The article section Inhibitors, poisons and promoters contains much more detail, and notes (with a source) that negative catalyst is an occasionnal synonym for inhibitor, but this does not need to be in the article intro. Dirac66 (talk) 02:17, 13 March 2013 (UTC)
"Complexogeneous Catalysis"
[edit]I vote that we remove any reference to so-called 'complexometric catalysis' in this article. It is not a concept which is widely recognised within the catalysis community, its description is poorly written and makes no sense, and there doesn't seem to be any reliable references for it. Throw it away. — Preceding unsigned comment added by ColloidsMan5000 (talk • contribs) 00:51, 13 March 2013 (UTC)
- I agree and removed this material, hopefully others will let my removal stand. Thanks for your comments. --Smokefoot (talk) 12:39, 13 March 2013 (UTC)
This is a new catalysis which can transfer biomass into standard fuel directly, since it can remove and transfer oxygen and hydrogen. You do not know that does not means it does not exist. Why you can remove my paper. — Preceding unsigned comment added by 108.173.197.74 (talk) 02:23, 5 April 2013 (UTC)
Someone remove my talk also. Why? If you think you are correct, please show up. Please do not underground to do something. I hope someone should have basic knowledge about catalysis, then can take part in talking. Liks Mr.ColloidsMan5000, can you told public people, if you have catalysis background or not? I will send message to public people to complain your behavior in this web. Even if you remove my message again, I will send complain on other web side. — Preceding unsigned comment added by Charleswang2601 (talk • contribs) 19:54, 6 April 2013 (UTC)
This web should let technical people to charge the message and give some contact information which can make a good communication. For example, if a catalysis expert to charge this area and have contact information. I can talk to him first to explain "complexogeneous" and show him newspaper, science article,production line video then put message on web. Otherwise, some children, like Mr.ColloidsMan5000, will say they do not know and they do not recognise. science will develop and go along. We can not only live in homogeneous/heterogeneous catalysis, which is invented by 50 years ago. — Preceding unsigned comment added by Charleswang2601 (talk • contribs) 20:06, 6 April 2013 (UTC)
I will put complexogeneous catalysis again on this web and give my real name and contact information there. I like to talk any catalytic experts if they have some question. Please do not remove my message again. Wikki web is to transfer good/correct message to public people.Thanks — Preceding unsigned comment added by Charleswang2601 (talk • contribs) 20:14, 6 April 2013 (UTC)
I have written a email to Viki web office to complain the person who make trouble on me. I want ViKi office to cancel this person's editor right. If you are "catalysis" person, please just show up to let's talk about catalysis. Underground to do something, is not like a gentleman. — Preceding unsigned comment added by 146.131.120.2 (talk) 19:32, 8 April 2013 (UTC)
Catalytic antibodies
[edit]Mentioned catalytic antibodies ("abzymes") in the section on emzymes and biocatalysts. Goblinshark17 (talk) 07:23, 14 September 2014 (UTC)
filter image
[edit]Would it be better if the image "Low Temperature Oxidation Catalyst.jpeg" be better displayed at a higher resolution and cropped? When I first saw it I thought it was vandalism because it looks like a sphere being held by a women. The hands take up too much of the picture. It was only when I clicked on the image that I could see the grid pattern. DGerman (talk) 19:40, 5 September 2015 (UTC)
recycled?
[edit]Can someone improve the sentence which states "Because catalysts are not consumed, they are recycled. "
The wikipedia article on recycling states: "Recycling is a process to convert waste materials i"
Perhaps something like "reusable"? DGerman (talk) 19:46, 5 September 2015 (UTC)
- I have written a longer phrase which is clearer although perhaps more awkward. I think that technically recycled is accurate as the molecule does enter a new catalytic cycle, but unfortunately recycled will for many readers suggest waste disposal as you have said, so it is better to reword the article. Dirac66 (talk) 02:09, 6 September 2015 (UTC)
External links modified
[edit]Hello fellow Wikipedians,
I have just modified 2 external links on Catalysis. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20080405195443/http://www.chem.umn.edu/services/lecturedemo/info/genie.htm to http://www.chem.umn.edu/services/lecturedemo/info/genie.htm
- Added
{{dead link}}tag to http://www.documentroot.com/2010/03/catalytic-antibodies-simply-explained.html - Added archive https://web.archive.org/web/20080517071700/http://www.climatetechnology.gov/library/2005/tech-options/tor2005-143.pdf to http://www.climatetechnology.gov/library/2005/tech-options/tor2005-143.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 22:11, 12 June 2017 (UTC)
The guidelines WP:TERTIARY and WP:SECONDARY
[edit]@Catalysis-for-all: Catalysis is a very, very big area. Citations to this topic should follow WP:TERTIARY (well recognized textbooks and monographs) or at least WP:SECONDARY (reviews). Data: 443940 references (journal articles, reviews, patents, etc), of which 181000 have appeared since 2009. That number translates to many papers per day. Hence the guidelines WP:TERTIARY and WP:SECONDARY. Hence the avoidance of individual specialized reports. Its impossible.--Smokefoot (talk) 11:07, 24 April 2019 (UTC)
Requested move 24 June 2021
[edit]- The following is a closed discussion of a requested move. Please do not modify it. Subsequent comments should be made in a new section on the talk page. Editors desiring to contest the closing decision should consider a move review after discussing it on the closer's talk page. No further edits should be made to this discussion.
The result of the move request was: not moved. There is a clear consensus that catalysis, not catalyst, best describes the topic of this article. (closed by non-admin page mover) Lennart97 (talk) 11:06, 1 July 2021 (UTC)
Catalysis → Catalyst – Catalyst was merged into Catalysis in 2006; the merge was warranted, however it should have gone to the article Catalyst instead of Catalysis. Catalyst is by far the more notable topic between the two: https://books.google.com/ngrams/graph?content=catalyst%2Ccatalysis&year_start=1800&year_end=2019&corpus=26&smoothing=3&direct_url=t1%3B%2Ccatalyst%3B%2Cc0%3B.t1%3B%2Ccatalysis%3B%2Cc0 . Furthermore, any discussion of catalysts must necessarily also include discussion catalysis, but the reverse is not necessarily true, and catalysts are at least as notable as catalysis in their own right, and likely more so. Firejuggler86 (talk) 15:11, 24 June 2021 (UTC)
- Oppose. Interesting suggestion, but my experience is that the topic of catalysis is far more general and more useful than catalyst. What can one say about "catalyst"? No generic catalyst exists. Zillions of individual catalysts are known but their only unifying aspect is that they catalyze reactions. No common structural or electronic principles describe them. Here is another way to think about this issue: no university course or a symposium would be entitled "Catalyst". No. But "Catalysis" or "Principles of Catalysis" or Heterogeneous Catalysis" are titles of classes throughout the world. And similar titles describe international symposia.--Smokefoot (talk) 16:33, 24 June 2021 (UTC)
- Oppose. Catalysis is a more general topic, and our article has sections which would not be well described by Catalyst. For example Mechanism refers to the reaction, not the substance. And Inhibitors, Poisons and Promoters are not Catalysts but rather other substances which modify the Catalysis mechanism. Dirac66 (talk) 18:40, 24 June 2021 (UTC)
- Oppose. I can see how this article might seem like it should be moved to catalyst. After all, the term catalyst appears 122 times in article as compared to the 49 times of catalysis. However, the article in question is specifically structured around the idea of catalysis, not catalysts in general. Catalysts are just individual substances. There are plenty of opportunities to expand upon other chemical articles about their use as catalysts without bogging down this article. Sir Trenzalore (talk) 21:50, 24 June 2021 (UTC)
- Oppose per the above. I agree an interesting idea, but the term 'catalysis' is a much more appropriate title for the content of the article. Mdewman6 (talk) 23:28, 24 June 2021 (UTC)
- oppose they are two separate things this article covers the process as a whole—catalysis—not more narrowly the catalyst substances—blindlynx (talk) 01:41, 25 June 2021 (UTC)
- Oppose I agree with others reasonings. This is fine as it currently is. --Tautomers(T C) 01:59, 28 June 2021 (UTC)
Catalytic reaction mechanisms
[edit]The first two paragraphs off this section are repetitious. 2603:6011:2903:C25A:7409:E4E2:21D1:3EEC (talk) 14:12, 13 April 2022 (UTC)
- C-Class vital articles
- Wikipedia level-3 vital articles
- Wikipedia vital articles in Physical sciences
- C-Class level-3 vital articles
- Wikipedia level-3 vital articles in Physical sciences
- C-Class vital articles in Physical sciences
- C-Class Chemistry articles
- Top-importance Chemistry articles
- WikiProject Chemistry articles