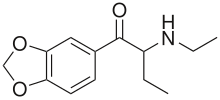
Eutylone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H17NO3 |
| Molar mass | 235.283 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Eutylone (also known as β-keto-1,3-benzodioxolyl-N-ethylbutanamine, bk-EBDB, and N-ethylbutylone) is a stimulant and empathogenic drug of the phenethylamine, amphetamine, phenylisobutylamine, and cathinone families which was developed in the 1960s,[3][4] which is classified as a designer drug.[5] It was first reported to the EMCDDA in 2014 and became widespread internationally in 2019-2020 following bans on the related compound ephylone.[6][7][8][9] It is a synthetic cathinone.[9] In 2021, eutylone was the most common cathinone identified by the Drug Enforcement Administration in the United States.[10]
Legal status
[edit]Sweden's public health agency suggested classifying eutylone as a hazardous substance, on September 25, 2019.[11]
In the United States Eutylone is considered a schedule 1 controlled substance as a positional isomer of Pentylone.[12][13]
See also
[edit]- 5-Methylethylone
- Butylone
- Ethyl-J
- Ethylone
- Ephylone
- N-Ethylhexedrone
- N-Ethylhexylone
- N-Ethylheptylone
References
[edit]- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ "Substance Details Eutylone". Retrieved 2024-01-22.
- ^ GB 108513, "Aryl-α-aminoketone derivatives", published 1967-09-27, assigned to Boehringer Ingelheim
- ^ Glatfelter GC, Walther D, Evans-Brown M, Baumann MH (April 2021). "Eutylone and Its Structural Isomers Interact with Monoamine Transporters and Induce Locomotor Stimulation". ACS Chemical Neuroscience. 12 (7): 1170–1177. doi:10.1021/acschemneuro.0c00797. PMC 9423000. PMID 33689284. S2CID 232192447.
- ^ "Eutylone". New Synthetic Drugs Database. 12 November 2023.
- ^ Bade R, White JM, Nguyen L, Tscharke BJ, Mueller JF, O'Brien JW, et al. (August 2020). "Determining changes in new psychoactive substance use in Australia by wastewater analysis". The Science of the Total Environment. 731: 139209. Bibcode:2020ScTEn.73139209B. doi:10.1016/j.scitotenv.2020.139209. PMID 32417485. S2CID 218680033.
- ^ Krotulski AJ, Papsun DM, Chronister CW, Homan J, Crosby MM, Hoyer J, et al. (August 2020). "Eutylone Intoxications-An Emerging Synthetic Stimulant in Forensic Investigations". Journal of Analytical Toxicology. 45 (1): 8–20. doi:10.1093/jat/bkaa113. PMID 33325503.
- ^ Chen HY, Chien WC, Huang MN, Fang CC, Weng TI (January 2021). "Analytically confirmed eutylone (bk-EBDB) exposure in emergency department patients". Clinical Toxicology. 59 (9): 846–848. doi:10.1080/15563650.2020.1868491. PMID 33448882. S2CID 231611658.
- ^ a b Nakamura M, Takaso M, Takeda A, Hitosugi M (September 2022). "A fatal case of intoxication from a single use of eutylone: Clinical symptoms and quantitative analysis results". Leg Med (Tokyo). 58: 102085. doi:10.1016/j.legalmed.2022.102085. PMID 35537301. S2CID 248602483.
- ^ "Emerging Threat Report" (PDF). 2021.
- ^ "Tretton ämnen föreslås klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 25 September 2019. Archived from the original on 31 October 2019. Retrieved 11 November 2019.
- ^ Lists of: Scheduling Actions. Controlled Substances. Regulated Chemicals
- ^ "Federal Register :: Request Access". 10 April 2023.
