Wikipedia talk:WikiProject Chemistry/Structure drawing workgroup/Archive 1
| This is an archive of past discussions about Wikipedia:WikiProject Chemistry. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 |
Software
Is there any free software that is accepted for drawing chemical diagrams for Wikipedia? Both of the programs recommended at Wikipedia:WikiProject_Chemistry/Structure_drawing#Suggested_molecule_editor_settings are proprietary. Superm401 - Talk 05:03, 21 December 2007 (UTC)
- Actually, you can draw diagrams in anything that will give you a good svg. I started out with inkscape, but that's a bit of a mission. I currently use BKchem. Also check out the list above.--Slashme (talk) 07:23, 21 December 2007 (UTC)
- Oh, and see the previous discussions at Wikipedia_talk:WikiProject_Chemistry/Structure_drawing_workgroup/Archive_index --Slashme (talk) 07:49, 21 December 2007 (UTC)
New image
I'd like to help producing some new .svg files (if I can be any use), but I'm having a few teething problems. I've uploaded a drawing (acepromazine), but It doesn't look quite right and I can't seem to get it to use the usual font for the atom labels. I'm using BKchem and Inkscape on OSX 10.5. Any suggestions would be appreciated. Thanks. David-i98 (talk) 02:26, 27 December 2007 (UTC)
Problems with SVG
I tried to contribute chemical structures as a SVG-file but unfortunately I did not succeed with any freeware program. I'm using ISIS/Draw to create the structure, safe in as WMF but did not find any way to properly convert it to SVG. With SVGfactory it works if there are no letters (C, H, N, O) present. With Inkscape the SVG-file just contains a link to the external file which is pretty useless. I tried several other programs, don't remember each one anymore. MarvinBeans from ChemAxon worked great to creat the SVG file but I could not change the settings to the wikipedia format. Can anyone recommend a simple solution, e.g. a free WMF to SVG converter? —Preceding unsigned comment added by Panoramix303 (talk • contribs) 11:15, 9 March 2008 (UTC)
- free structure drawing: BKchem. Exports directly to svg (Cairo!), no need for conversion tools. Have already done dozens of images with it V8rik (talk) 17:58, 9 March 2008 (UTC)
- I tried BKChem with the settings from the german structure drawing group (http://de.wikipedia.org/wiki/Wikipedia:Wie_erstelle_ich_Strukturformeln%3F#Ma.C3.9Fe) but there are several problems, e.g. the orientation of hydrogens can't be changed, after importing a mol-file every implicit hydrogen has to be added by hand.... So the whole solution is not really convenient.
-- Panoramix303 (talk) 19:25, 9 March 2008 (UTC)
Freeware solution for SVG files
I had serious problems getting proper SVG files with freeware/open source software. Here is a step-by-step guide how to do it, hopefully it helps other people.
1) Draw the structure in any program which can export WMF-files (e.g. ISIS/Draw or ChemDraw)
2) Export the files as WMF
3) Download the file wmftosvg.exe (http://www.argentum.freeserve.co.uk/wmftosvg.exe)
4) Go the command prompt (cmd.exe)
5) Go the directory of wmftosvg.exe
6) type: wmftosvg.exe filename.wmf >filename.svg
7) open the created filename.svg with an texteditor
8) add two lines after the line <svg version....."
9) first line: width=""
10) second line: height=""
11) between the "" add the width and height of the wmf, the values can be seen in the properties tab after right-clicking on the file
12) safe the edited svg-file
13) DONE!
Sounds complicated but takes less than two minutes to finish everything, no need to abandon the loved structure editor and no need for big and/or complicated programs.
-- Panoramix303 (talk) 19:36, 13 March 2008 (UTC)
- That seems very interesting. How did you find this .exe file? Fvasconcellos (t·c) 20:07, 13 March 2008 (UTC)
Category
The category Chemical structure quality issues, in which the project page is supposed to be placed, doesn't exist: any ideas for a better category? Physchim62 (talk) 13:59, 29 March 2008 (UTC)
Broken Link
This link: http://www.iupac.org/publications/pac/2006/pdf/7810x1897.pdf
doesn't exist anymore. —Preceding unsigned comment added by 66.92.19.253 (talk) 21:13, 13 March 2008 (UTC)
- I just clicked on the link that you gave, and got a PDF. Check again? --Slashme (talk) 15:21, 16 May 2008 (UTC)
Need a check
Created Carvonic acid, but needs a check if I did it right.--Stone (talk) 18:15, 15 May 2008 (UTC)
- Looks OK to me. Now it should be easy to make one for Carvone as well ;) --Slashme (talk) 15:23, 16 May 2008 (UTC)
Getting started, need feedback
OK, I'm trying out this SVG thing using BKChem, Inkscape, and Mysid's script. I randomly picked out a PNG marked as "needs to be in SVG" and redrew it. The original is here and this is my version. Could someone take a look at it and let me know what needs improvement? Thanks! --JaGa (talk) 09:22, 9 July 2008 (UTC)
Hi Jaga, welcome to Wikichem!
Image looks perfect, thanks! --Rifleman 82 (talk) 10:42, 9 July 2008 (UTC)
those pesky hydrogens
So, I'm puttering away on making SVGs. I'm seeing in skeletal diagrams including stereochemistry that hydrogens are shown where a ring carbon joins a chain, like here. Make sense, I suppose, since in the diagram the orientation of the other atoms is represented in the plane. Do we have a policy one way or another? Personally, I like leaving the hydrogens Image:Nonactin.svg out because it's less cluttered, but I'd rather fix a couple of images now than a bunch later. What are your thoughts? (BTW isn't the symmetry of nonactin cool?) --JaGa (talk) 12:02, 11 July 2008 (UTC)
- Yep, pretty cool symmetry! In this case, by leaving out all those hydrogens, you're losing the stereochemical information at those positions. In some of those positions, you can draw a wedge for the side-chain, but not in all of them, so you'll have to put in at least a few hydrogens, as far as I can tell. --Slashme (talk) 12:34, 11 July 2008 (UTC)
- (edit conflict) There isn't any policy one way or another, but in general hydrogen atoms with stereochemistry should be drawn explicitly where the dashed and wedged bonds would otherwise be within a ring (as in steroids). The way that Image:Nonactin.svg is currently drawn, there is no defined stereochemistry at the chiral centers where the ring carbons join the chain, so the image as drawn at the fermentek site is better. If you want to instead define the stereochemistry by putting dashed and wedged C-C bonds going away from the ring, that would be OK, but not ideal because of potential confusion in the four cases where there is an adjacent chiral center bearing a methyl group. (I hope this makes sense - it's hard to discuss stereochemistry without being able to point to something.) And, yes, the symmetry of nonactin is quite cool. -- Ed (Edgar181) 12:41, 11 July 2008 (UTC)
- Great comments guys, and thanks. I put the necessary hydrogens in. (new version) I want to get to the point where I can look at a molecule, know where the explicit hydrogens should be, and when/how I can move bonds to make explicit hydrogens unnecessary. I knew all this once, but it's slow in the remembering. Thanks again for explaining with patience and friendliness. --JaGa (talk) 14:33, 11 July 2008 (UTC)
Best representation of 5,5'-Azotetrazolate salt
I'm not experienced with drawing salts, but I was planning on doing G2ZT. I have an idea of the structure, thanks to Stone, but am not sure how to depict the negative charges. I found this image of a different 5,5'-Azotetrazolate salt. Is that the best way - with the circle containing a negative sign in each ring, or, could I show 2-3 and 4-5 double bonds on each tetrazole and place a negative sign on each tetrazole's #1 nitrogen? --JaGatalk 19:54, 25 August 2008 (UTC)
- I like the way it is represented in the link you give - the circles are a good way of indicating that the negative charges are well distributed around the ring. -- Ed (Edgar181) 20:02, 25 August 2008 (UTC)
- Yeah, I tend to agree. Here's the finished product.
Colored atoms in structures
I am wondering if we have a position for or against color-coded atoms in chemical structures and if we should add a sentence about this to the guideline?
I personally do not like color coded structures much because they limit the use outside Wikipedia, they do not add additional information, they are not common in the chemical literature (with the exception of certain introductory text books), and for me the colors are somewhat distracting. Cacycle (talk) 18:07, 13 September 2008 (UTC)
- I assume you're talking about 2D structures? Ball&stick and other 3D models are pretty difficult for non-experts to interpret without color-coding. I agree that color on stick figures can be distracting and doesn't add any information to the structure itself. However, it can be useful for matching the stick-figure skeletal drawing to a 3D structure that is color-coded the same way (see Caffeine infobox for a great example--IMO, and also that the FA folks liked as well if I recall). An analogous question is whether chemical formulas should be color-coded by element. I find that even more distracting, especially because colored-text has UI meaning (clickable link, previously-visited link, etc). That's not just a hypothetical...other places the different elements within a formula are wikilinked to the elements' pages. But again, if you're not familiar with skeletal diagrams, color can help figuring out what's what (but again-again, everything except C and some H are labeled, so it doesn't really help much).
- I think the coloring the stick-figure diagrams also make them less useful outside of WP and teaching settings, since it might suggest a meaning that isn't intended (if the reuser uses certain colors to highlight certain features, reuser would first have to uncolor everything). I guess it all comes down to a balance between "looking nice" and being as useful as possible to the lay public vs being concise and non-distracting for more advanced users. I'd say coloring is fine for infobox or other places where it parallels other colored images, but not in general. DMacks (talk) 18:38, 13 September 2008 (UTC)
- Sorry, I am talking only about 2D chemical formulas such as this one:
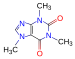 3D should always be color coded.Cacycle (talk) 19:01, 13 September 2008 (UTC)
3D should always be color coded.Cacycle (talk) 19:01, 13 September 2008 (UTC)
- Sorry, I am talking only about 2D chemical formulas such as this one:
Personally, I prefer colors on large molecules  , but it looks distracting on small molecules, although I will use it on small molecules if it helps illustrate an article, like here in the Federal Analog Act. I don't think it's really an issue unless we use the image in articles that put several images side by side. And of course, the reuse outside of WP is less of an issue for SVGs, since you could make any image all black with a text editor in seconds. --JaGatalk 07:54, 14 September 2008 (UTC)
, but it looks distracting on small molecules, although I will use it on small molecules if it helps illustrate an article, like here in the Federal Analog Act. I don't think it's really an issue unless we use the image in articles that put several images side by side. And of course, the reuse outside of WP is less of an issue for SVGs, since you could make any image all black with a text editor in seconds. --JaGatalk 07:54, 14 September 2008 (UTC)
Best way to draw Anisodamine
Looking for suggestions - how would you deal with this? The nitrogen is really oddly placed; it looks like implied chirality with normal lines. I'd prefer to draw it more flat with chiral lines, but don't want to make an assumption that could be wrong, and am not even positive how I'd do it. What are your thoughts? --JaGa (talk) 04:06, 18 July 2008 (UTC)
- Oops, just saw your post. I'd expect the nitrogen to be achiral due to Walden inversion. If you disagree, feel free to remove my image.
- If you *have* to define chirality (or for a phosphorus), why not have a bond to a lone pair? Looks better than having a lone pair balloon. Either way, you can define it that way? --Rifleman 82 (talk) 05:36, 18 July 2008 (UTC)
- No worries! Your version looks better than PubChem's, no way I would want to change that. Now I'm off to learn about Walden inversions ;) --JaGa (talk) 05:39, 18 July 2008 (UTC)
The wiki article doesn't talk about nitrogen flipping. Wikibooks says something about it, other sources describe the flipping, but not explicitly as a Walden inversion. My memory says that the lone pair can exchange positions via a trigonal planar intermediate with the 2 electrons in a p orbital above and below the plane. Don't take my word for it! --Rifleman 82 (talk) 05:52, 18 July 2008 (UTC)
- If you look at it from an NMR point of view, depending on the solvent, amine protons are acidic and undergo exchange *anyway*. So you'll get scrambling of the original chirality, if any. --Rifleman 82 (talk) 05:56, 18 July 2008 (UTC)
- Wrong inversion! See Nitrogen inversion, though I usually call it "pyramidal inversion". (A Walden inversion is a set of chemical reactions designed to convert a chiral carbon center into its opposite form - a different thing.) I like to see the inversion of a nitrogen as N going from sp3 to a flat (achiral) sp2, then back to sp3 (in the opposite form). Anyway, Rifleman's image looks better than Pubchem's, but the benzylic position still has unspecified chirality - is that right? ChemSpider shows both forms, as well as the unspecified entry (from PubChem?). This may need checking in the primary literature. Cheers, Walkerma (talk) 06:28, 18 July 2008 (UTC)
The image at PubChem is missing the stereochemistry on the side chain - it should be (S), not (R/S), according to Chemical Abstracts. I also don't really like the way PubChem depicts the endo/exo geometry at the stereocenter bearing the hydroxy group. Perhaps the image can be drawn similar to the way scopolamine or hyoscyamine are depicted. -- Ed (Edgar181) 11:41, 18 July 2008 (UTC)
- All the tropanes have the same easy-to-draw-confusingly chain attachment issue. Cocaine looks pretty clear. DMacks (talk) 12:29, 18 July 2008 (UTC)
In this particular case the inversion is a non-issue. From nitrogen inversion: However, if the nitrogen is a bridgehead atom in a bicyclo or a similar compound where it cannot invert around the lone electron pair, then the nitrogen atom could be a chiral center. Therefore anisodamine can have that chiral nitrogen drawn out as it is. Nice pic by the way! --WhirlwindChemist (talk) 12:43, 1 November 2008 (UTC)
- Actually, in this case, the nitrogen is not at a bridgehead position and it can invert. -- Ed (Edgar181) 13:05, 1 November 2008 (UTC)
Explaining Skeletal Structures
After looking at a couple of pages such as caffeine, where we have gradient coloured atoms in the infobox structure, and seeing non-chemists confused by the implicit presence of hydrogens in skeletal structures, I was wondering if we could address this somehow. Would it be reasonable to have some sort of "What does this mean?/About these structures" link associated with the structures to give an explanation to non-specialists as to how/why we draw as we do. This could be a link to skeletal structure, or some basic version of this pointing out the main concepts. One single picture showing implicit H's, Me as a 'line', standard atom colours, * for chiral centre, isotope labels, etc. might be useful and improve accessibility. Any thoughts??? --WhirlwindChemist (talk) 13:06, 1 November 2008 (UTC)
As an early example, which could have arrows pointing out relevant info:
--WhirlwindChemist (talk) 13:36, 1 November 2008 (UTC)
- See CPK coloring for the coloring standard. (I think we should be using these colors on all diagrams.) --Arcadian (talk) 15:56, 1 November 2008 (UTC)
I don't think line diagrams should use any colors *at all*. I know that pubchem does like to use coloring, but ... Color should be used only for ball and stick or spacefill diagrams where the atoms are not explicitly labeled. --Rifleman 82 (talk) 16:33, 1 November 2008 (UTC)
- I personally agree that line diagrams should be B&W only. Unless I was producing something where it was essential to draw attention to something, e.g. to help explain a convoluted catalytic cycle, then there is no reason to use coloured atoms. However, having said that we do have structure images, such as that for caffeine, which even have localised gradient colouring that expands beyond the atom onto the bond. My point in this particular discussion is not whether this should be allowed (my personal opinion is that it is unnecessary, and some colours are notoriously hard to see on the standard wiki-white background), but that what conventions we/wikipedia do have are explained quickly to non-specialists. The diagram above is merely what I could think of in two minutes on ChemDraw, and I offer it as only a very basic example just to illustrate the idea. Thanks for your thoughts guys, and is there anywhere or way we can get a bigger discussion going on the colours issue (as started in the section above)? I can foresee though that non-specialists will find colour helpful/less boring/colourful (sorry for that one!) etc. and thus grounds for contention. --WhirlwindChemist (talk) 13:13, 2 November 2008 (UTC)
- What about having a left-aligned "Chemical structure:" link on top of the structure, similar to all the other fields such as "Molecular formula:"? Additional 3-D structure could get a "Space-filling model:" link. Cacycle (talk) 15:35, 2 November 2008 (UTC)
Explicit methyl hydrogens
Hello everyone,
As far as I remember, WP:CHEM tends to prefer explicit methyl groups (i.e. R-CH3 instead of R—).
This is not mentioned in the Manual of Style (at least I don't think it is).
The MoS says:
- "hydrogens should be implied (hidden), except for the benefit of the target audience"
and this is causing some confusion.
I propose that the MoS is updated to say:
- "hydrogens should be implied (hidden), except in the case of methyl groups or for emphasis or the benefit of the target audience".
The preference for explicit methyl groups could also be discussed in the same place that it says:
- "If desired, the methyl group should be denoted with "CH3", not "Me" due to possible confusion with the German abbreviation for metal ("Metall")"
Ben (talk) 00:36, 20 May 2009 (UTC)
- Sorry I was slow to reply - just saw this, as I was away when you commented. I'm in general agreement with your proposal. There are two types of cases where this situation can arise:
- Personally I like the explicit methyl in case #1, but not in #2, even though Nicolaou has tried to popularise the latter. What do others think? Walkerma (talk) 03:25, 12 June 2009 (UTC)
- I have the same preference as Martin. --Itub (talk) 12:48, 12 June 2009 (UTC)
Yes, this makes sense. I didn't really think about methyls at the end of longer alkyl chains - i just considered them to be, e.g. hexyls!
Very important point, I agree with you both.
Ben (talk) 23:09, 12 June 2009 (UTC)
- p.s., another case, 3, is that of methyl groups bonded to heteroatoms, e.g. in amines such as RNMe2. I would use skeletal methyls (1) sometimes, (2) always and (3) rarely if ever.
- Funny, I just drew one in IsisDraw a few minutes ago! My answer would be #3, though I could live with #1. My impression is that skeletal methyls in such cases are pretty rare in the literature. Walkerma (talk) 04:22, 13 June 2009 (UTC)
- Sorry, I don't think I explained myself clearly. What I meant was, my preference is:
- (1) methyl groups coming off a chain or ring - use skeletal methyl groups sometimes, if it looks better in context
- (2) methyl groups terminating alkyl chains - use skeletal methyl groups always
- (3) methyl groups bonded to heteroatoms - rarely if ever use skeletal methyl groups
- Looks good to me! Walkerma (talk) 03:25, 14 June 2009 (UTC)
- Hy Ben, I agree with your preferences (1) to (3). However, I'm also a supporter of Nicolaou: methyl group that terminates a longer chain (e.g., in octane should be drawn with an explicit methyl group. --Jü (talk) 10:24, 21 June 2009 (UTC)
![]() Done See Wikipedia:Manual of Style (chemistry)/Structure drawing. Physchim62 (talk) 13:03, 21 June 2009 (UTC)
Done See Wikipedia:Manual of Style (chemistry)/Structure drawing. Physchim62 (talk) 13:03, 21 June 2009 (UTC)
Table on settings in different programs
What do you think about adding a table such as the one in de:WP:WEIS#Maße that lists the suggested settings in different programs? --Leyo 11:53, 27 September 2010 (UTC)
- We used to have one, several years ago now. Then we did a study on optimizing the settings, and found that ACS settings were just as good as anything we could invent, and far easier to explain to new users. Maybe this needs to be made clearer in our guidelines. Physchim62 (talk) 12:57, 27 September 2010 (UTC)
- That would also be an option. We have sentences like “ACS templates are available in most chemical drawing programs.”, but the do not jump into the reader's eye. :-) --Leyo 14:53, 27 September 2010 (UTC)
Want to contribute
Long time ago since I edited an article seriously.
I want to contribute again. This time, I want to do some chemistry to keep me interested. I think that the best for me is helping drawing compounds and reactions because it will cost me least time. I may do more in the summer holiday or so. The question is where I should start. Thanks.
Georginho (talk) 22:10, 26 March 2011 (UTC)
- Wikipedia:WikiProject Chemistry/Image Request might be a good starting point. --Leyo 21:03, 27 March 2011 (UTC)
- Very nice. Will take a look into that in foreseeable future.
- Georginho (talk) 03:47, 28 March 2011 (UTC)
JChemPaint Applet - apparently not appropriate
Attempts to use the JChemPaint Applet at http://www.ebi.ac.uk/steinbeck-srv/jchempaint-3.0.1/EditorApplet.html to produce a structure for acefluranol and save as a PNG image file resulted in too tight cropping of the structure, chopping at least one methyl group in half (this was using applet in Chrome). Saving file to SVG format and opening in Firefox showed neither F nor O atoms properly, defaulting everything to non-alphanumeric and, thus, incorrectly to carbons. If others have had success, useful to know this. --User:Ceyockey (talk to me) 00:56, 29 March 2011 (UTC)
- I tried and had the same problem with the PNG image.
- Georginho (talk) 19:01, 1 April 2011 (UTC)
Preferred structure style - multiple groups associated
In the case of Calcium bicarbonate two valid structure types are possible:
 or
or 
Which would be generally more favored by this group? --User:Ceyockey (talk to me) 12:21, 2 April 2011 (UTC)
- As far as I know, WP:CHEM has no preference. Different editors prefer/use different styles.
- I personally tend to opt for the bracket style, because it's analogous to the text formula Ca(HCO3)2. No–one writes HCO3CaO3CH, but it's different for structural images.
Ball-and-Stick vs. Spacefilling vs. Line
There is prevalent use of all three of the structure types among images associated with chemical articles. The choice of best practice is not specified in Wikipedia:Manual of Style (chemistry)/Structure drawing (as far as I could see). My feeling is that a Line-format structure should be included in an article which sports a ball-and-stick or spacefilling image, preferably in the Chembox. This has no doubt been discussed before . . . what was the outcome of that discussion? Thanks --User:Ceyockey (talk to me) 20:49, 2 April 2011 (UTC)
- from User:ceyockey — The article Ammonium bisulfate illustrates what the preferred solution should be, in my opinion. --20:52, 2 April 2011 (UTC)
- We include a structural formula (what you call a "line-format structure") in almost all cases. A ball-and-stick model is included as well if (i) the structure is known, (ii) someone has made an image of sufficient quality and correctness, and (iii) such a 3D would benefit the reader. Space-filling models are usually less desirable because it's harder to see each atom (many are obscured) and thus harder to discern the finer points of the overall structure. Nonetheless, SF models are useful for indicating molecular volume and steric bulk.
- We do tend to choose which images to include on a case-by-case basis. An important example of deviation from the general rule I above is simple network solids like sodium chloride. NaCl's structure seems to be most clearly illustrated by a space-filling model. There is a ball-and-stick (and polyhedral) representation further down the article at Sodium chloride#Crystal structure. No "line-format structure" is provided, probably because it would be messy. See Commons:Category:Crystal structure of sodium chloride for a selection of similar representations.
Dots vs lines for nonbonded electrons
Is there a preference or standard between these two ways of representing explicit lone-pairs? Lewis structure seems to distinguish lines-as-bonds vs dots-as-nonbonded, which is the only thing I ever really see now in recent publications (either primary-literature and textbooks). But I see a lot of WP:COMMONS and other diagram sources that use a line to represent a pair in both contexts. I find it confusingly similar to a "formal-charge minus" designation and/or confusingly similar to a bond when there are lots of both of them in a congested area of a diagram. Actually, I can't find a current ref indicating this non-bonded use at all, let alone as a current-use standard. DMacks (talk) 16:40, 9 April 2011 (UTC)
- Germans tend to represent lone pairs as lines. I think it's common in a few other European countries. I prefer pairs of dots for all the reasons you give, plus I like what I grew up with.
Polymers
WP:CSDG does not contain a guideline on how polymers are best drawn. For the depiction of repetation units, there are several options:
- Brackets: square, round or curly
- n: normal or italic
- Bonds between repetition units: solid or dashed lines
This issue is currently discussed in de-WP. What are the opinions here? --Leyo 12:32, 23 November 2012 (UTC)
- My personal preference is square brackets, normal, and solid.
- I usually draw polymers by drawing the first end, the repeating unit, and the other end. It's the most convenient because 1) it's a polymer and 2) it's directly done with ChemDraw: selecting the repeating unit, selecting Object - Add Frame - Brackets, right-clicking the right bracket and Bracket Usage - SRU (n), and editing "n" with the Text tool to change it to a number if necessary.
- But of course, 1) we have random polymers and 2) I have no idea how to draw polymers with other programs.
- Georginho (talk) 22:47, 1 October 2013 (UTC)
- The relevant document by IUPAC is probably DOI:10.1351/pac199466122469 that says:
- square or round brackets
- “n” italic
- solid lines
- --Leyo 08:29, 2 October 2013 (UTC)
- The relevant document by IUPAC is probably DOI:10.1351/pac199466122469 that says:
- Thanks. It looks good to me.
- I looked further, and for cyclic polymers, they seem to use only square brackets: http://dx.doi.org/10.1351/pac200880020201. So, I think I would propose 1) square brackets, 2) italic "n", but normal "number", and 3) solid lines.
- Georginho (talk) 09:35, 2 October 2013 (UTC)
R S configuration

I found the images commons:File:R S configuration.png (used at absolute configuration) and its vectorization commons:File:R S configuration.svg (pictured) demonstrate the chirality very confusingly. The depiction of the bond towards D is non-standard and, more importantly, this visual arrangement is ambiguous about whether D, X, and CR/AS are collinear or not. Compare it with good images, for example: commons:File:Carbone_asymetrique.png and commons:File:Chiral_sym_CHXYZ.svg. Could somebody advise: what should I alter in Chiral_sym_CHXYZ.svg to obtain two distinct images for R- and S- as the replacement for this R_S_configuration crap? (The thing similar to what I already made with commons:File:Amine N-R.svg and commons:File:Amine R-N.svg.) Incnis Mrsi (talk) 13:57, 16 February 2014 (UTC)
Representing charges
I think we do need an agreement on this, related to the line-vs-dot discussion above. Ozonolysis has both charges and charges-within-circle.
I heard that Americans like charges, while Europeans charges-within circle. Is this true?
I personally prefer no circle. I think it will also be consistent if we draw charges within the text in the article. But I'm also fine with circle.
Thoughts?
Georginho (talk) 22:25, 3 October 2013 (UTC)
- Charges within circles. Reasoning is as long as it is clear, but in part, the symbol for a negative charge is too easily varied, and too bloody easy to lose in a large scheme. Two standard and scalable images need to be created, that can be incorporated into drawings. LeProf — Preceding unsigned comment added by 50.179.245.225 (talk) 22:50, 23 February 2014 (UTC)
Why ACS settings
Is the only reason because these settings are widely available? I would prefer longer bond lengths. J1812 (talk) 22:42, 29 January 2013 (UTC)
- Can you refer to an example compound, where you think the ACS setting do not look nice? --Leyo 09:35, 30 January 2013 (UTC)
- Yes. Here is a mechanism I'm drawing next to diagram from the textbook source - http://imgur.com/SGJ82GU As you can see, the arrow is a tight squeeze. Do you think users would mind? J1812 (talk) 08:18, 2 February 2013 (UTC)
I would like to second J1812's objection! I have been victimised for the simple fact that I love MarvinSketch and it's svg 2D structural images and I don't see anything wrong with them. So what if they're not ACS? How is that so problematic? Fuse809 (talk) 15:50, 30 September 2013 (UTC)
- There is no perfect style. And everybody has his or her preference regarding which style is the nicest. If you use ChemDraw, I would say that the ACS style is the clearest. The Wiley style comes close, but the labels are sometimes so big that you don't see the bond in a clear way. That is, I guess, why people in general like the ACS style. But even the ACS style isn't convenient for presentations, in my opinion and personal preference. For presentations, I modify it. But still, it's a good style to begin your modifications. (For fun, e.g., during a course where I had to handed in reports for experiments, I used different styles for each report.) I think we the Wikipedians choose the ACS style because yeah, it looks the nicest at the moment.
- If you want to draw mechanisms, I agree that the bonds are sometimes to short. What I do is just elongating the bonds. I got no complain so far; so, I think my pictures are good. :)
- I made two so far:
- https://en.wikipedia.org/wiki/Acetalization
- https://en.wikipedia.org/wiki/Tiffeneau%E2%80%93Demjanov_rearrangement
- I think there is and should be always a room for flexibility in drawing chemical structures. Disabling "Fixed Angles" in ChemDraw is useful to draw the arrows, in case you didn't know.
- Georginho (talk) 22:24, 1 October 2013 (UTC)
- By the way Fuse809, I just saw your talk page. You've indeed been under the scope by others for your images. I understand that you use MarvinSketch. And I think the pictures are fine. (Although I would like bolder bonds and bigger labels.)
- But I do agree with the others that we need some consistency. We agreed before that the ACS style is the one we will be using. We also display the settings in the manual page so that it's easier for users using programs without default settings.
- Your thoughts are welcome.
- Georginho (talk) 22:39, 1 October 2013 (UTC)
- While agree there is no perfect style, I disagree strongly with arguments up to this point in the chat that might be construed as suggesting that variability in representation is acceptable in the long term. Think of the pedagogic issues that would be raised by a textbook that did not use a consistent drawing convention beginning to end. And that audience—unsophisticated, like readers of textbooks—is the fundamentally core of our encyclopedia audience. See my closing arguments (three points and postscript), as of this date, below. LeProf — Preceding unsigned comment added by 50.179.245.225 (talk) 22:55, 23 February 2014 (UTC)
Racemates

- WP:CSDG currently states "For articles on racemates, only one enantiomer should be depicted ordinarily". However according to IUPAC,[1]: 1968 the preferred way to depict a racemate is side by side figures as in the figure to the right. The current WP:CSDG guideline in my opinion is wrong since drawing a racemate as only one enantiomer implies that it is optically pure which it clearly is not. The most precise depiction of a racemate is side by side figures as IUPAC recommends. However this becomes rather verbose if a reaction sequence is drawn out. Here the usual convention is to use a wiggly bond (mixture) or unspecified stereochemistry to represent racemates. I propose to change the wording of WP:CSDG to following the IUPAC recommendation when just a single structure is drawn or use wiggly bonds in reaction schemes. Thoughts? Boghog (talk) 10:47, 3 April 2015 (UTC)
- I'm surprised by both our guidelines and by IUPAC's. CSDG's instructions to depict one enantiomer doesn't make sense as written - it seems to suggest drawing with one enantiomer specified, which would be misleading because that is the typical way of indicating that it is not a racemate. By far the most common practice here on Wikipedia for racemates (with only one stereocenter) is not to specify the stereochemistry, such as in file:Amphetamin.svg. Less commonly, the wavy bond is used like in file:Amphetamine-2D-skeletal.svg. Almost never do we use the side by side figures like File:Racemic amphetamine.svg. This overall practice is consistent with what is used by the organic chemistry community outside Wikipedia such as in textbooks or organic chemistry journals. Unspecified stereochemistry is the norm for racemates. In my experience, the side by side representation as recommended by IUPAC is only used in specific situations where two enantiomers are explicitly being compared in some way. In Wikipedia infoboxes, the only cases where side by side images make sense, in my opinion, is where there is more complicated stereochemistry - such as in tramadol. I don't think it would be a good idea to change the wording of CSDG to following the IUPAC recommendation when just a single structure is drawn or use wiggly bonds in reaction schemes, because that is not common practice in the chemistry community or what is commonly done here. -- Ed (Edgar181) 11:36, 3 April 2015 (UTC)

- Thanks for your response. I agree that the most common way to depict a racemate is to draw the stereochemistry undefined as in the figure to the right. Furthermore some of IUPACs recommendations are rather strange including this one (see also MOSCHEM editing policy which advises not to rigidly follow IUPAC recommendations if they deviate from common usage). Hence I agree that we should follow common practice and not IUPAC in this case. So I propose we change CSDG's wording to:
- "For articles on racemates, the stereochemistry should be depicted as undefined (straight and not wavy nor wedged bonds attached to the stereogenic center)." Boghog (talk) 12:12, 3 April 2015 (UTC)
- Thanks for your response. I agree that the most common way to depict a racemate is to draw the stereochemistry undefined as in the figure to the right. Furthermore some of IUPACs recommendations are rather strange including this one (see also MOSCHEM editing policy which advises not to rigidly follow IUPAC recommendations if they deviate from common usage). Hence I agree that we should follow common practice and not IUPAC in this case. So I propose we change CSDG's wording to:
Since you two support the use of unspecified stereochemistry for images of racemates, I went ahead and updated MOS:CSDG according to Boghog's wording. Nearly every depiction of the compound in the amphetamine article uses the straight instead of the wavy line for the methyl group at the alpha carbon. I also figure that it's simpler to just make the MOS compliant with the article than to redraw everything to make the article compliant with the MOS (I am indeed that lazy) - that article requires MOS compliance because it's featured. ![]() Seppi333 (Insert 2¢ | Maintained) 05:39, 5 April 2015 (UTC)
Seppi333 (Insert 2¢ | Maintained) 05:39, 5 April 2015 (UTC)
References
- ^ Brecher, Jonathan (31 January 2006). "Graphical representation of stereochemical configuration (IUPAC Recommendations 2006)" (PDF). Pure and Applied Chemistry. 78 (10): 1897–1970. doi:10.1351/pac200678101897.
Marvinsketch
I have two questions, firstly, are we aloud to use fonts aside from arial (but still sans-serif) in MarvinSketch? Secondly, are why does MarvinSketch's down wedge orientation have to be daylight? I've created structures in ACS style using ChemSketch that had their bonds oriented downwards (MDL). Fuse809 (talk) 11:59, 10 March 2014 (UTC)
I have a question about the recommended "Wireframe bond thickness: 0.06". It's barely visible. 3.00 seems to work just fine and looks similar to most other structures.Aethyta (talk) 19:40, 17 June 2015 (UTC)
WP:CSDG listed at Redirects for discussion

An editor has asked for a discussion to address the redirect WP:CSDG. Please participate in the redirect discussion if you have not already done so. Deryck C. 15:31, 1 December 2016 (UTC)
There is currently and RFC on what do do with the shortcuts used for the chemistry-related projects. Please comment. Headbomb {talk / contribs / physics / books} 16:13, 14 February 2017 (UTC)
Structure quality control in organic chemical drawing
Points, three in number (for the moment):
(1) No re-inventing the wheel; the Evans-Harvard or a Nicolaou-Scripps CD templates should be used for organic chemical drawing. These templates are inspired by the Corey revolution in production (of new chemistry and so of new images), and in German pedagogy (if one looks back far enough); they are used everywhere in America, in part because of the wide and deep impact that these organic training programs have had on organic pedagogy in the US. (I did my training at Illinois and the University of Chicago, and the templates we used were slight touch-ups to the Harvard template.) This is both because there are long traditions and good chemical reasonings for the decisions that have gone into these templates, and because an otherwise sound chemical structure that appears in other formats makes chemical perception harder for the least trained readers in the community (and communicates to those well-trained, that the creator might be outside the mainstream of chemical training). Is there really any argument against uniformity of representation, for the sake of our readers?
(2) The best way to get this done would be to facilitate organic structure drawing, from a locked template AT WIKIPEDIA. We create text from scratch here, why not chemical images? I have negotiated gratis or near-gratis provisions for such tools at not-for-profits, knew at last checking at least some of the individuals making such decisions, and would be glad to try to engage the software providers to try to support a chemical drawing environment at Wikipedia. (The biggest hurdle to my personal completion of chemical projects here, is the hurdle that is placed to chemical drawing import. Moving it to a completely separate technology and workflow hinders the creative and technical process of creating text and images that go hand in hand.) Is anyone well enough placed within the organization to see such a massive "sea change" in how we do things? I would rather someone introduced me to the right Wikipedia manager/executive, etc., than to cold call and attempt to establish trust from scratch.
(3) This matter raised in response to the "charges in circles, or charges and no circles" discussion. Whatever is done, the overall matter of chemical representation has to be settled cohesively, and consistently, and once-and-for-all. The policy cannot evolve—this ignores tens to hundreds of years of past discussions and decisions by the leaders in the field, as well as the normalization of practices pursued by IUPAC. No solving problems one at a time. If we cannot start from a template here, we need to have thorough IUPAC and standard practice-based set of decisions in place, and an approved web-downloadable template, so poor drawings can be replaced that are in articles, better images can go into new articles, and (if necessary) images can eventually be rejected for format as well as fundamental content (just as, at times, portions of text are)—all because we have clear policies about chemical representation.
Bottom line, Wikipedia continues to view chemical representation simply as a subset of Wikimedia graphical content. However, as Roald Hoffmann has rightly argued, our representations are actually another formal language, and Wikipedia needs to move past seeing these as graphics, and facilitate our use of this universal language, on the sites. LeProf
- I would note in postscript that this broad argument comes after spending many hours in the English and German Wikipedias, and at Wikimedia, trying to find the best chemical representations for the articles to which I contribute, and despite the time spent and training/rigour ("eye") applied, the images in the articles are generally poor. (My editing time does not allow for the wasted time of back and forth between drawing and text generation environments I argue against, above.) For an example of the pedagogic issues, distinct but related to the foregoing, see the aminoglycoside article which I have begun to try to improve. Note the array of representations and poses of the amino glycoside structures in the gallery. This is not at all helpful to non-specialists. Then, note ones ability to look for subtle changes when the structures are uniform in representation and pose (e.g., within the series of chair representations.) And despite its current mediocre quality (it was worse before recent attention), I cannot address these issues fully. I simply haven't the time, every time a representation/pedagogy issue is found, to leave Wikipedia, go to CD Ultra, and create a starting structure from scratch (think brevetoxin, not nitrocubane), and then elaborate that into the series of precise structures needed. However, if a structure existed that I could open and edit, as I do text, the efficiency and amount achievable would vastly increase. LeProf
I can highly appreciate this is a difficult issue. Some of the structures .... well they really cannot be seen in the browser; they are too small. I am not a fan of the ACS style for this reason. theslave (talk) 00:45, 22 June 2017 (UTC)
Updating drawing guidelines to modern standards
The existing article on drawing chemical structures is very out-of-date. It refers to Accelrys Draw and Symyx Draw, both of which no longer exist, being replaced by BIOVIA Draw, still available to many as a free download "No-fee BIOVIA Draw for Academic and Non-commercial Use". Retrieved 2020-06-14.. Another package not mentioned is ACD/Chemsketch, which is also freely available. "ACD/ChemSketch for Academic and Personal Use". Retrieved 2020-06-14.. Both these chemistry editors can export .png graphics directly or be used in combination with other free software such as Inkscape or Libre Office Draw to convert formats they do support (such as .emf) into Wikipedia-preferred .svg files. Both support InChi and can open MDL's original ISIS/Draw (now non-proprietary) .mol format that can be downloaded from Chemspider for a vast range of existing chemicals, as a start point for many drawings.
I propose to re-write this section of MOS:CSDG and perhaps add a "how-to" worked example. Before I do that, do others have views on what should or should not be incorporated? Michael D. Turnbull (talk) 17:28, 14 June 2020 (UTC)
 Done I have updated the page to mention ACD/ChemSketch and BIOVIA Draw, removing the now obsolete Accelrys Draw and SymyxDraw. Michael D. Turnbull (talk) 12:12, 14 August 2020 (UTC)
Done I have updated the page to mention ACD/ChemSketch and BIOVIA Draw, removing the now obsolete Accelrys Draw and SymyxDraw. Michael D. Turnbull (talk) 12:12, 14 August 2020 (UTC)
Open Babel
What about open babel? Wouldn't the promotion of open source software for drawing structures be more in line with the philosophy of wikipedia? http://openbabel.org/docs/current/FileFormats/Overview.html Ethan Bass (talk) 22:08, 26 October 2020 (UTC)
- Open Babel is indeed a useful tool worthy of mention (it's a bluelink, so it's "notable" in the wikipedia sense). Translates from a zillion formats. But I don't know how it's very useful for drawing in particular. The mediawiki software does not support any of the chemistry formats, only plain graphics. There have been some attempts to get 3D formats supported, which would be a huge asset for crystal structures and such. DMacks (talk) 22:36, 26 October 2020 (UTC)
MarvinSketch part outdated (Manual of Style/Chemistry/Structure drawing)
The information on MarvinSketch is no longer applicable. Some of the settings are no longer where they used to be, and some are missing completely or have been renamed (double bond spacing). Also no units are given, which makes it especially difficult. — Preceding unsigned comment added by Naturwiki (talk • contribs) 05:20, 5 November 2020 (UTC)
- I haven't used MarvinSketch in years. Do you know where the settings are and other details to help update the information? DMacks (talk) 05:24, 5 November 2020 (UTC)
Transparent .png / .svg visibility (or lack thereof) in "night mode" browsers
Hi,
This applies to both structures and whatever markdown is generating chemistry equations, as well as all the mathml on wikipedia (but nowhere else on the web that I've seen thus far, since most CMS that support it use downloadable fonts styled like the rest of the page, or HTML5 canvas set to apply styles).
So far in both Samsung Internet (chrome based i believe) and Firefox, the majority of structures are invisible or almost so. This setting is pretty much required in order to conserve battery on modern phones with OLED displays as well as avoiding burn-in of both phone and retinas longer term.
There's not much to be done about .png that's uploaded, although a 50% grey instead of near black would fix it for my case without significant graphics changes. A non-transparent .png would be even better. An out of place white background is less offensive than unreadable splotches. Since user-supplied background color / stylesheet overrides have been around since the 90s at least this isn't exactly new, but there's less motivation to use a black / dark background on my desktop. OTOH I'm not a web designer but I'm almost certain that .svg can be styled inline. I'm not sure why wikipedia doesn't just do font-based rendering for formulas, since the 3 computers on earth running something archaic enough to not support it are going to get hardlocked by a 2004 zero-day before they finish loading a page.
In any case, the entire chemistry project, pharmacology, and math are partly inaccesible without changing browser settings back to day mode in what has to be the more common use case than machines with no svg or unicode support, and it's pretty annoying (not to mention bad design practices); unless the user has forced text and background to black because of insanity, the page should be displayable. Every other site manages it, so why not here? What does wikipedia do for text-only browsers / screen-readers for these. Obviously the structures have less options but i thought pointing out the reactions might give somebody an idea... I sadly don't know enough about how things are generated here to delve into solutions.
Cheers, --A Shortfall of Gravitas (other machine) (talk) 06:06, 22 March 2021 (UTC)
Default BIOVIA Draw ACS template
Hello, it has come up in my recent discussion with @Mike Turnbull that the ACS template from BIOVIA Draw doesn't match the ACS style guidelines. The most relevant setting being that the bond length of 1 cm should be adjusted down to 0.508 cm for a 10 pt font. This would match the recommended ACS settings for ChemDraw (courtesy link). There may be other settings that require adjusting if conflicts end up being created, as I'm not familiar with the software.
In many scenarios this does not affect the quality of the diagram, except when scaling down the image/svg there is a tendency for the heteroatom labels to become illegible past a certain point.
I'm bringing the issue here on Mike Turnbull's advice to consider adding a note to the MOS:CSDG page regarding recommended settings. Synpath (talk) 23:15, 25 November 2022 (UTC)

- My discussion with Synpath came about as we were involved in the GA review for thiamine, an article where I have supplied most of the diagrams. I use Biovia Draw, with its settings in the provided ACS style, converting to .svg in Inkscape. My version of Biovia Draw uses a file called "ACS Document.xml" dated 20 September 2017 for its journal settings. However, as Synpath has convinced me, this is not producing the recommended rendering. See this comparison for strychnine in ChemSketch with their ACS setting:
- The Biovia version is quite legible when introduced as above but becomes very poor by comparison when seen as a thumbnail. So.... 1) Are my findings correct (I may have messed something up)? 2) How should we advise others via WP:CSDG to set Biovia Draw?
- Alerting Walkerma, Smokefoot, Graeme Bartlett, DMacks and Leyo as some currently active editors who may have views. Mike Turnbull (talk) 15:54, 28 November 2022 (UTC)
- Many of these figures are displayed in thumbnail sizes in infoboxes, so the thumbnail legibility is important. Hence I think the Biovia font should be bolded and increased in size. Boghog (talk) 18:30, 28 November 2022 (UTC)
- I agree the Biovia image has text that is too small in comparison to the bond sizes. I don't think "bold" is the answer, as that can have a different purpose in some diagrams. Can those who use these pieces of software dig out the actual setting that they claim are "ACS"? If they give different visual results then either they have different actual settings (and one or both aren't actually the ACS spec) or they do not actually give results matching the settings (and one or both has a substantive bug in that regard). DMacks (talk) 18:39, 28 November 2022 (UTC)
- The ACS Style Guide (mentioned in the original post here) specifies among other things bond-length 14.4 pt (0.2 in.) and text 10 pt, and that this is the ChemDraw "ACS 1996" style. Per the style guide, "authors using other drawing packages should adapt these parameters to their systems", so it seems like Biovia's default is not correct. DMacks (talk) 18:52, 28 November 2022 (UTC)
- On my talk page Mike linked me a screenshot of their Biovia session with ACS settings on display. While not the entirety of the xml doc it has the most relevant settings, and it looks like the diagrams are accurately reproduced after converting to SVG. (Google Drive link) Synpath (talk) 19:14, 28 November 2022 (UTC)
- @DMacks This is a link to a copy of the Biovia .xml file (it is < 1 kb). I'm not competent to say how this translates into the settings shown in my other Google Drive link provided by Synpath. I've joined the Biovia online community in the hope I can get some insight from them. I'll also be investigating alternative settings I can use in Biovia Draw to try to find some that work as well as the ChemSketch ones illustrated above seem to do. Mike Turnbull (talk) 12:24, 29 November 2022 (UTC)
- OK, all, mea culpa. I've sorted this out now and the issue was at my end. It turns out that it is very easy to accidentally alter the actual settings used within Biovia Draw to move away from the defaults. Any changes the user makes get saved in a personal profile and at some time in the past I did mess up. Having restored the correct defaults today, you can see from the updated images here that the ChemSketch and Biovia "ACS" settings give very nearly identical output. Mike Turnbull (talk) 14:28, 29 November 2022 (UTC)
- I blame Professor Pebkac. DMacks (talk) 15:59, 29 November 2022 (UTC)
- I see this has been resolved now - thanks, Mike, for figuring this out. However, I wanted to mention one related problem I've seen a lot with Biovia Draw. I've been using this program almost daily now for 30 years (originally as IsisDraw) - I wrote my dissertation with it - and I have found that if you open older IsisDraw or even some Accelrys Draw files in Biovia Draw, often the font size appears wrong. The software claims it's (say) 10pt but it appears as 12 pt. So just be careful when using older files for new things! Cheers, Walkerma (talk) 05:08, 30 November 2022 (UTC)
- I blame Professor Pebkac. DMacks (talk) 15:59, 29 November 2022 (UTC)
- OK, all, mea culpa. I've sorted this out now and the issue was at my end. It turns out that it is very easy to accidentally alter the actual settings used within Biovia Draw to move away from the defaults. Any changes the user makes get saved in a personal profile and at some time in the past I did mess up. Having restored the correct defaults today, you can see from the updated images here that the ChemSketch and Biovia "ACS" settings give very nearly identical output. Mike Turnbull (talk) 14:28, 29 November 2022 (UTC)
- I agree the Biovia image has text that is too small in comparison to the bond sizes. I don't think "bold" is the answer, as that can have a different purpose in some diagrams. Can those who use these pieces of software dig out the actual setting that they claim are "ACS"? If they give different visual results then either they have different actual settings (and one or both aren't actually the ACS spec) or they do not actually give results matching the settings (and one or both has a substantive bug in that regard). DMacks (talk) 18:39, 28 November 2022 (UTC)
- Many of these figures are displayed in thumbnail sizes in infoboxes, so the thumbnail legibility is important. Hence I think the Biovia font should be bolded and increased in size. Boghog (talk) 18:30, 28 November 2022 (UTC)



