Wikipedia:Reference desk/Archives/Science/2017 June 18
Appearance
| Science desk | ||
|---|---|---|
| < June 17 | << May | June | Jul >> | June 19 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
June 18
[edit]Francium
[edit]Why is francium so unstable? The article doesn't explain. Google found me a Prezi presentation ([1]), but first off that's not a reliable source for expanding the francium article, and secondly it doesn't explain why many isotopes of related elements, e.g. 238U with 54 more neutrons than protons, are so much longer lived. Nyttend (talk) 12:31, 18 June 2017 (UTC)
- Island_of_stability has some general info on how people have tried to understand stability of stuff with high atomic numbers. I don't think it gives a direct and simple answer to why francium has a short half life, but lots of good relevant information and theory is presented. SemanticMantis (talk) 15:33, 18 June 2017 (UTC)
- Additional good stuff at Semi-empirical mass formula, valley of stability, and magic number_(physics). The magic numbers and semi-empirical bits lead to to believe that a complete and thorough answer in terms of first principles may be beyond our current understanding though I'd be happy to see refs to the contrary! SemanticMantis (talk) 16:37, 18 June 2017 (UTC)
- At a low level, it isn't very surprising - I mean, it's not like technetium sitting in the middle of the periodic table. It comes in a rogues gallery near the tail end of the known stable isotopes, between astatine and radon on one side and radium and actinium on the other. There is one little clump of stable isotopes after that - thorium, protactinium, uranium, neptunium, plutonium (protactinium and the latter two aren't all that stable, but at least you can take a picture of a big lump of plutonium, though somebody might have to kill you afterward). Wnt (talk) 17:21, 18 June 2017 (UTC)
- Minor note: you noted that uranium-238 has 54 more neutrons than protons. Yes, that's what makes it relatively stable. Neutrons, just like protons, attract other nucleons through the nuclear force, but, since they're electrically neutral, they don't repel other neutrons or protons. Hence, neutrons are essential for stabilizing nuclei with higher numbers of nucleons. The neutron–proton ratio of stable isotopes goes up with increasing atomic number, for this reason. --47.138.161.183 (talk) 21:24, 18 June 2017 (UTC)
- That is absolutely not the full story, or else heavier uranium isotopes would be even more stable. You need to take beta decay into account; too few neutrons and a few protons will want to turn into neutrons to increase binding energy, but too many neutrons and some will want to tun into protons for the same reason. In the middle we have the line of beta stability, a sort of "Goldilocks zone" for the nucleus. There are also regions of increased stability against other decay modes: here alpha decay and spontaneous fission are also important.
- In the case of thorium and uranium, the beta-stable and alpha-stable lines coincide, and nuclides like 232Th, 235U, and 238U have not much of a desire to decay. Astatine and francium have a big problem because the lines do not coincide in that region: you will find that the most stable isotopes of At and Fr towards one decay mode quite readily suffer the other one.
- Admittedly this explanation just puts the question one step further back. Why does the beta-stability line dive into a region of alpha instability just past Pb and Bi? The reason is because doubly magic 208Pb has a very stable closed nuclear shell, so much so that the energy gap between the highest occupied and lowest unoccupied proton and neutron shells is very big. Alpha decay thus ends up releasing enough energy and being energetically feasible, and thus if you look at a chart of nuclides the alpha-decay region appears to suddenly explode from polonium onwards. (Why Po and not Bi? Because making the alpha particle from Bi requires breaching the full subshell for both protons and neutrons. 210Po is less inhibited because emitting the alpha gets us back to the filled proton shell, and because both start and end nuclei are zero-spin; it is easier to form the alpha when the nucleons that form it are already paired the right way.) The same thing happens, though with much longer half-lives, in the lanthanide series once the closed shell of 82 neutrons is surpassed.
- As a result, stability is greatly depressed until Th and U with their semi-closed shells at 142 neutrons and 92 protons. (Already the alpha- and beta-stability lines are not quite in sync; the most stable isotopes 232Th, 238U, and 244Pu can suffer double beta decay, and from Cm onwards spontaneous fission from the overly large Coulomb repulsion of protons puts an end to the island. But the region of alpha-stability is less skewed to the neutron-rich side than it is around astatine and francium.) Double sharp (talk) 06:31, 19 June 2017 (UTC)
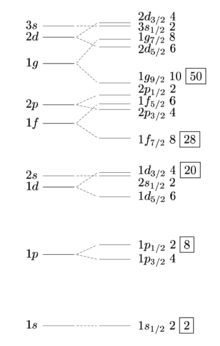
- @Double sharp: One thing that bothers me about the magic numbers and closed shells is that they seem to emerge from a nuclear shell model that gives what, to my untrained eye, seems like a kind of random and unpredictable set of energy levels. I mean, the diagram I just put at right doesn't go high enough to cover the shells closed in lead, but from the text I gather it closes all the shells with n=6 or less, plus it fills the highest-momentum n=7 state, so the location of the magic number in the periodic table shows a pattern. But ... why are the next energy states so much higher in energy? If you get all the way out to n=184, do we know that gets low-energy enough that an island of stability returns? Why or why not? Articles like this make it sound like even the location is up for grabs, let alone whether there are truly stable elements there. The light speed constraints throw a huge wild card into the mix. And then, as that article also points out, there are heavier isotopes of a lot of known elements whose stability hasn't been tested, and may be longer. (I also wonder how much of that is known but being kept secret because they make nuclear initiators or very small nuclear weapons out of them) I don't feel like someone has a computer program that can just tell you what the half-life of an isotope ought to be based on some Schroedinger-like equation, and in the absence of that, saying that elements are less stable after lead because a shell has been filled almost seems like a tautology. Wnt (talk) 23:03, 23 June 2017 (UTC)
- @Wnt: The nuclear-initiator theory has a big problem: such isotopes should decay by spontaneous fission instead of alpha decay. Hence they would tend to act like 240Pu and cause the bomb to fizzle quite quickly, which is not what you would want at all.
- The trouble with half-life calculations is that they not only are very complicated, but the best heuristics for doing them require a lot of fine-tuning of parameters. Using the same parameters through the whole chart of nuclides may be morally laudable but will give you lacklustre results, so people doing predictions in different areas of the nuclide chart generally have to tweak the parameters. If you are trying to discover things about the known regions, all you are doing is a lengthly and very silly exercise in interpolation.
- The next energy states being much higher in energy is kind of like how you go from noble gas to reactive alkali metal on the periodic table; a shell is filled and the next electron has to go into the next higher s-orbital. Much the same thing happens with the nucleus, except that the one after Pb (unlike, say the one after Sn) results in a much higher energy gap (because finally the strong force is at its limits), so the decay ends up taking a rather short time compared to that of, say, 147Sm. Double sharp (talk) 06:49, 25 June 2017 (UTC)
- @Double sharp: I remember that right after the Pokhran-II tests, the Indian DAE was shown celebrating in a picture (I think it might be in here) where there were three scientists, one holding up two fingers, the next five, the next one. Perhaps coincidentally, californium-251 is an isotope that doesn't decay by spontaneous fission. It's hard to say what unknown isotopes don't decay by SF. ;) Now to be sure, as I go up our articles like isotopes of hassium and such, I do see a lot more completeness than I was thinking there was, and it's hard to believe there is a Cf-251 for every isotope going up the chart when the others are so closely decaying. And yet, there are still a few left out, and I can always look suspiciously on what mysterious wonders might be hidden from us by well-chosen gaps... Wnt (talk) 09:34, 25 June 2017 (UTC)
- @Double sharp: One thing that bothers me about the magic numbers and closed shells is that they seem to emerge from a nuclear shell model that gives what, to my untrained eye, seems like a kind of random and unpredictable set of energy levels. I mean, the diagram I just put at right doesn't go high enough to cover the shells closed in lead, but from the text I gather it closes all the shells with n=6 or less, plus it fills the highest-momentum n=7 state, so the location of the magic number in the periodic table shows a pattern. But ... why are the next energy states so much higher in energy? If you get all the way out to n=184, do we know that gets low-energy enough that an island of stability returns? Why or why not? Articles like this make it sound like even the location is up for grabs, let alone whether there are truly stable elements there. The light speed constraints throw a huge wild card into the mix. And then, as that article also points out, there are heavier isotopes of a lot of known elements whose stability hasn't been tested, and may be longer. (I also wonder how much of that is known but being kept secret because they make nuclear initiators or very small nuclear weapons out of them) I don't feel like someone has a computer program that can just tell you what the half-life of an isotope ought to be based on some Schroedinger-like equation, and in the absence of that, saying that elements are less stable after lead because a shell has been filled almost seems like a tautology. Wnt (talk) 23:03, 23 June 2017 (UTC)

- @Wnt: Usually the even-even ones are expected to be SF-decaying, since there is little hindrance (whereas the unpaired spin of an odd proton or neutron makes SF less likely). So if you look at the Hs isotope table, you see only the heavy odd isotopes get listed, and I think it's rather safe to expect that the even ones are a lot more short-lived. Regarding decay modes, predictions have been done, as you can see on the right. ^_^
- The problem with making isotopically pure 251Cf is that there isn't really an obvious way to separate it from 250Cf and 252Cf, which will be produced simultaneously, and do decay by SF (and they are also quite dangerous because of its shorter half-lives). Uranium isotopes can be separated because UF6 is volatile, but Cf is essentially always stuck in the +3 oxidation state and has no known volatile compounds. So off the top of my head there does not seem to be a very practical way to do this yet. Double sharp (talk) 09:58, 25 June 2017 (UTC)
- @Double sharp: I wasn't really thinking about how it would be made, nor do I really know that much about it, though I'd think that the Iranians with their ultracentrifuges could separate just about any isotope. Alternatively, I see the isotopes of fermium include Fm-255 which is more stable than most - I'd think if someone had a way to make that without so much Fm-252, then they could wait around half a day, purify the results chemically, and wait for the Cf-251 to arrive.
- But the table of predicted decay modes is indeed interesting ... the question is, how well have the real results matched against the predictions? And there's a circled "island of stability" on that chart, but how stable do they mean? Wnt (talk) 14:00, 25 June 2017 (UTC)

- @Wnt: AFAIK separation with centrifuges still needs a gaseous or at least volatile compound like UF6 to do the work, so for Cf it is not very good. And while you can get 255Fm rather simply by going up the neutron-capture path, the difficulty is that you get less and less material as you go up element by element; by the time Fm is reached you are making only picograms, and you will get only picograms of 251Cf out, nowhere near enough.
- The real results have so far matched very well; the only mismatch with what we see now is the prediction of some electron capture branches (marked in red) that haven't been seen yet – and more recent results seem to indicate that we may have just gotten lucky the first time around in not seeing them, because we seem to be seeing the indirect results of them now (we still have no way of detecting EC in superheavy isotopes, because the detectors are made for finding alphas). As for the stability, there is also a predicted lifetime chart: the longest half-lives predicted are for 291Cn and 293Cn of about 1200 years (references at copernicium). Double sharp (talk) 14:12, 25 June 2017 (UTC)
- I'm afraid to say that I've been deluded quite a while on one point -- when I read the Iranians were using ultracentrifuges, I assumed they were doing something like a cesium chloride gradient in solution! I was thinking the light isotopes would rise to the top, maybe with some comparably charged ion added in solution of just the right density to tend to separate the two isotopes out a bit more once chemistry was done ... ah, nevermind. I see from [2] that indeed the centrifugation was another gas based method. We ought to have an article about it, but what I've seen (you can tell) have been news stories obviously lacking in detail.
- It seems like much of what is here, the tables, the reference in copernicum, and some of the other ideas, all traces back to a Dubna review paper. Interestingly, they appear hopeful that they can get to the island of stability (such as it is) by a succession of beta+/electron capture decays if they can just get *one* more neutron into the mix first. I guess close counts in horseshoes, hand grenades, and nuclear science. ;) But some of the other stuff, like using multiple nuclear bombs in close succession to get superheavy elements, or making a pulsed fission reactor with a thousand times bigger pulses ... wow. Good thing Russia is on the far side of the world. ;) Wnt (talk) 16:22, 25 June 2017 (UTC)
Yellowstone national park super explosion
[edit]Would the eclipse which could be total in Wyoming put enough gravity on the hot magnma underneath yellowstone and make supervolcanos to destroy America? Can the fracking make it even worse? Thankyou! 64.134.238.170 (talk) 18:26, 18 June 2017 (UTC)
- Total eclipses visible in Wyoming have, probably, happened multiple times since the last eruption 630,000 ago without any consequences. Ruslik_Zero 18:29, 18 June 2017 (UTC)
- No. Absolutely not. A geographic location is totally eclipsed every few centuries on average. I don't know about fracking. And I'm on the East Coast but Wikipedia's news ticker is on my watchlist and I saw "Yellowstone national park super explosion" new section and 1 reply and was like oh my God. Sagittarian Milky Way (talk) 19:02, 18 June 2017 (UTC)
- And even if it could, there's nothing to be done about it, so worrying is fruitless. ←Baseball Bugs What's up, Doc? carrots→ 19:20, 18 June 2017 (UTC)
- No, because eclipses do not have a significant effect on gravitional effects on Earth. It's just not fundamentally different from any other spring tide. Even then, the maximal effect is a perigean spring tide, which occurs three or four times every year (far more frequent than eclipses), and the August 2017 eclipse does not coincide with one. The maximal totality duration for August 2017 is about 2:40, substantially less than the possible maximum of around 8 minutes of totality. Finally, the inclination of the lunar orbit with respect to Earth (about 5°) means that even when the moon is maximally angled away from directly overhead, some 99.6% of the theoretical maximal gravity pull is still oriented along the vertical — that's the key bit to explain why the fact that it's an eclipse (and directly overhead) simply doesn't matter for gravitational purposes. — Lomn 19:26, 18 June 2017 (UTC)
- Also note that the solar eclipse of March 9, 2016 did not trigger an eruption of Lake Toba. Count Iblis (talk) 22:53, 18 June 2017 (UTC)
- But wouldn't the tides suck more molten rock into the caldera? Scientists seem to be unconcerned because the caldera is only 10% molten rock, but tides could increase that. 144.35.114.222 (talk) 16:51, 20 June 2017 (UTC)
