Beta-decay stable isobars
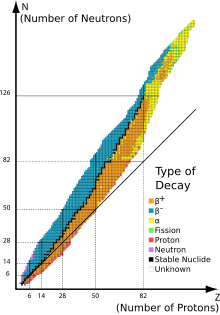
Beta-decay stable isobars are the set of nuclides which cannot undergo beta decay, that is, the transformation of a neutron to a proton or a proton to a neutron within the nucleus. A subset of these nuclides are also stable with regards to double beta decay or theoretically higher simultaneous beta decay, as they have the lowest energy of all isobars with the same mass number.
This set of nuclides is also known as the line of beta stability, a term already in common use in 1965.[1][2] This line lies along the bottom of the nuclear valley of stability.
Introduction
[edit]The line of beta stability can be defined mathematically by finding the nuclide with the greatest binding energy for a given mass number, by a model such as the classical semi-empirical mass formula developed by C. F. Weizsäcker. These nuclides are local maxima in terms of binding energy for a given mass number.
| βDS | One | Two | Three |
|---|---|---|---|
| 2-34 | 17 | ||
| 36-58 | 6 | 6 | |
| 60-72 | 5 | 2 | |
| 74-116 | 2 | 20 | |
| 118-154 | 2 | 12 | 5 |
| 156-192 | 5 | 14 | |
| 194-210 | 6 | 3 | |
| 212-262 | 7 | 19 | |
| Total | 50 | 76 | 5 |
All odd mass numbers have only one beta decay stable nuclide.
Among even mass number, five (124, 130, 136, 150, 154) have three beta-stable nuclides. None have more than three; all others have either one or two.
- From 2 to 34, all have only one.
- From 36 to 72, only eight (36, 40, 46, 50, 54, 58, 64, 70) have two, and the remaining 11 have one.
- From 74 to 122, three (88, 90, 118) have one, and the remaining 22 have two.
- From 124 to 154, only one (140) has one, five have three, and the remaining 10 have two.
- From 156 to 262, only eighteen have one, and the remaining 36 have two, though there may also exist some undiscovered ones.
All primordial nuclides are beta decay stable, with the exception of 40K, 50V, 87Rb, 113Cd, 115In, 138La, 176Lu, and 187Re. In addition, 123Te and 180mTa have not been observed to decay, but are believed to undergo beta decay with an extremely long half-life (over 1015 years). (123Te can only undergo electron capture to 123Sb, whereas 180mTa can decay in both directions, to 180Hf or 180W.) Among non-primordial nuclides, there are some other cases of theoretically possible but never-observed beta decay, notably including 222Rn and 247Cm (the most stable isotopes of their elements considering all decay modes). Finally, 48Ca and 96Zr have not been observed to undergo beta decay (which is theoretically possible for both), but double beta decay is known for both.
All elements up to and including nobelium, except technetium, promethium, and mendelevium, are known to have at least one beta-stable isotope. It is known that technetium and promethium have no beta-stable isotopes; current measurement uncertainties are not enough to say whether mendelevium has them or not.
List of known beta-decay stable isobars
[edit]350 beta-decay stable nuclides are currently known.[3][4] Theoretically predicted or experimentally observed double beta-decay is shown by arrows, i.e. arrows point towards the lightest-mass isobar. This is sometimes dominated by alpha decay or spontaneous fission, especially for the heavy elements. Possible decay modes are listed as α for alpha decay, SF for spontaneous fission, and n for neutron emission in the special case of 5He. For mass 5 there are no bound isobars at all; there are bound isobars for mass 8, but the beta-stable one 8Be is unbound.[5]
Two beta-decay stable nuclides exist for odd neutron numbers 1 (2H and 3He), 3 (5He and 6Li – the former having an extremely short half-life), 5 (9Be and 10B), 7 (13C and 14N), 55 (97Mo and 99Ru), and 85 (145Nd and 147Sm); the first four cases involve very light nuclides where odd-odd nuclides are more stable than their surrounding even-even isobars, and the last two surround the proton numbers 43 and 61 which have no beta-stable isotopes. Also, two beta-decay stable nuclides exist for odd proton numbers 1, 3, 5, 7, 17, 19, 29, 31, 35, 47, 51, 63, 77, 81, and 95; the first four cases involve very light nuclides where odd-odd nuclides are more stable than their surrounding even-even isobars, and the other numbers surround the neutron numbers 19, 21, 35, 39, 45, 61, 71, 89, 115, 123, 147 which have no beta-stable isotopes. (For N = 21 the long-lived primordial 40K exists, and for N = 71 there is 123Te whose electron capture has not yet been observed, but neither are beta-stable.)
All even proton numbers 2 ≤ Z ≤ 102 have at least two beta-decay stable nuclides, with exactly two for Z = 4 (8Be and 9Be – the former having an extremely short half-life) and 6 (12C and 13C). Also, the only even neutron numbers with only one beta-decay stable nuclide are 0 (1H) and 2 (4He); at least two beta-decay stable nuclides exist for even neutron numbers in the range 4 ≤ N ≤ 160, with exactly two for N = 4 (7Li and 8Be), 6 (11B and 12C), 8 (15N and 16O), 66 (114Cd and 116Sn, noting also primordial but not beta-stable 115In), 120 (198Pt and 200Hg), and 128 (212Po and 214Rn – both very unstable to alpha decay). Seven beta-decay stable nuclides exist for the magic N = 82 (136Xe, 138Ba, 139La, 140Ce, 141Pr, 142Nd, and 144Sm) and five for N = 20 (36S, 37Cl, 38Ar, 39K, and 40Ca), 50 (86Kr, 88Sr, 89Y, 90Zr, and 92Mo, noting also primordial but not beta-stable 87Rb), 58 (100Mo, 102Ru, 103Rh, 104Pd, and 106Cd), 74 (124Sn, 126Te, 127I, 128Xe, and 130Ba), 78 (130Te, 132Xe, 133Cs, 134Ba, and 136Ce), 88 (148Nd, 150Sm, 151Eu, 152Gd, and 154Dy – the last not primordial), and 90 (150Nd, 152Sm, 153Eu, 154Gd, and 156Dy).
For A ≤ 209, the only beta-decay stable nuclides that are not primordial nuclides are 5He, 8Be, 146Sm, 150Gd, and 154Dy. (146Sm has a half-life long enough that it should barely survive as a primordial nuclide, but it has never been experimentally confirmed as such.)
| Even N | Odd N | |
|---|---|---|
| Even Z | Even A | Odd A |
| Odd Z | Odd A | Even A |
| Odd A | Even A | Odd A | Even A | Odd A | Even A | Odd A | Even A |
|---|---|---|---|---|---|---|---|
| 1H | 2H | 3He | 4He | 5He (n) | 6Li | 7Li | 8Be (α) |
| 9Be | 10B | 11B | 12C | 13C | 14N | 15N | 16O |
| 17O | 18O | 19F | 20Ne | 21Ne | 22Ne | 23Na | 24Mg |
| 25Mg | 26Mg | 27Al | 28Si | 29Si | 30Si | 31P | 32S |
| 33S | 34S | 35Cl | 36S ← 36Ar | 37Cl | 38Ar | 39K | 40Ar ← 40Ca |
| 41K | 42Ca | 43Ca | 44Ca | 45Sc | 46Ca → 46Ti | 47Ti | 48Ti[a] |
| 49Ti | 50Ti ← 50Cr | 51V | 52Cr | 53Cr | 54Cr ← 54Fe | 55Mn | 56Fe |
| 57Fe | 58Fe ← 58Ni | 59Co | 60Ni | 61Ni | 62Ni | 63Cu | 64Ni ← 64Zn |
| 65Cu | 66Zn | 67Zn | 68Zn | 69Ga | 70Zn → 70Ge | 71Ga | 72Ge |
| 73Ge | 74Ge ← 74Se | 75As | 76Ge → 76Se | 77Se | 78Se ← 78Kr | 79Br | 80Se → 80Kr |
| 81Br | 82Se → 82Kr | 83Kr | 84Kr ← 84Sr | 85Rb | 86Kr → 86Sr | 87Sr | 88Sr |
| 89Y | 90Zr | 91Zr | 92Zr ← 92Mo | 93Nb | 94Zr → 94Mo | 95Mo | 96Mo ← 96Ru[b] |
| 97Mo | 98Mo → 98Ru | 99Ru | 100Mo → 100Ru | 101Ru | 102Ru ← 102Pd | 103Rh | 104Ru → 104Pd |
| 105Pd | 106Pd ← 106Cd | 107Ag | 108Pd ← 108Cd | 109Ag | 110Pd → 110Cd | 111Cd | 112Cd ← 112Sn |
| 113In | 114Cd → 114Sn | 115Sn | 116Cd → 116Sn | 117Sn | 118Sn | 119Sn | 120Sn ← 120Te |
| 121Sb | 122Sn → 122Te | 123Sb | 124Sn → 124Te ← 124Xe | 125Te | 126Te ← 126Xe | 127I | 128Te → 128Xe |
| 129Xe | 130Te → 130Xe ← 130Ba | 131Xe | 132Xe ← 132Ba | 133Cs | 134Xe → 134Ba | 135Ba | 136Xe → 136Ba ← 136Ce |
| 137Ba | 138Ba ← 138Ce | 139La | 140Ce | 141Pr | 142Ce → 142Nd | 143Nd | 144Nd (α) ← 144Sm |
| 145Nd | 146Nd → 146Sm (α) | 147Sm (α) | 148Nd → 148Sm (α)[c] | 149Sm | 150Nd → 150Sm ← 150Gd (α) | 151Eu (α) | 152Sm ← 152Gd (α) |
| 153Eu | 154Sm → 154Gd ← 154Dy (α) | 155Gd | 156Gd ← 156Dy | 157Gd | 158Gd ← 158Dy | 159Tb | 160Gd → 160Dy |
| 161Dy | 162Dy ← 162Er | 163Dy | 164Dy ← 164Er | 165Ho | 166Er | 167Er | 168Er ← 168Yb |
| 169Tm | 170Er → 170Yb | 171Yb | 172Yb | 173Yb | 174Yb ← 174Hf (α) | 175Lu | 176Yb → 176Hf |
| 177Hf | 178Hf | 179Hf | 180Hf ← 180W (α) | 181Ta | 182W | 183W | 184W ← 184Os (α) |
| 185Re | 186W → 186Os (α) | 187Os | 188Os | 189Os | 190Os ← 190Pt (α) | 191Ir | 192Os → 192Pt |
| 193Ir | 194Pt | 195Pt | 196Pt ← 196Hg | 197Au | 198Pt → 198Hg | 199Hg | 200Hg |
| 201Hg | 202Hg | 203Tl | 204Hg → 204Pb | 205Tl | 206Pb | 207Pb | 208Pb |
| 209Bi (α) | 210Po (α) | 211Po (α) | 212Po (α) ← 212Rn (α) | 213Po (α) | 214Po (α) ← 214Rn (α) | 215At (α) | 216Po (α) → 216Rn (α) |
| 217Rn (α) | 218Rn (α) ← 218Ra (α) | 219Fr (α) | 220Rn (α) → 220Ra (α) | 221Ra (α) | 222Ra[d] (α) | 223Ra (α) | 224Ra (α) ← 224Th (α) |
| 225Ac (α) | 226Ra (α) → 226Th (α) | 227Th (α) | 228Th (α) | 229Th (α) | 230Th (α) ← 230U (α) | 231Pa (α) | 232Th (α) → 232U (α) |
| 233U (α) | 234U (α) | 235U (α) | 236U (α) ← 236Pu (α) | 237Np (α) | 238U (α) → 238Pu (α) | 239Pu (α) | 240Pu (α) |
| 241Am (α) | 242Pu (α) ← 242Cm (α) | 243Am (α) | 244Pu (α) → 244Cm (α) | 245Cm (α) | 246Cm (α) | 247Bk (α) | 248Cm (α) → 248Cf (α) |
| 249Cf (α) | 250Cf (α) | 251Cf (α) | 252Cf (α) ← 252Fm (α) | 253Es (α) | 254Cf (SF) → 254Fm (α) | 255Fm (α) | 256Cf (SF) → 256Fm (SF) |
| 257Fm (α) | 258Fm (SF) ← 258No (SF) | [e] | 260Fm[f] (SF) → 260No (SF) | [g] | 262No (SF) |

All beta-decay stable nuclides with A ≥ 209 are known to undergo alpha decay, though for some, spontaneous fission is the dominant decay mode. Cluster decay is sometimes also possible, but in all known cases it is a minor branch compared to alpha decay or spontaneous fission. Alpha decay is energetically possible for all beta-stable nuclides with A ≥ 165 with the single exception of 204Hg, but in most cases the Q-value is small enough that such decay has never been seen.[12] With the exception of 262No, no nuclides with A > 260 have been definitively identified as beta-stable. 260Fm is unconfirmed.[10] Moreover, the known beta-stable nuclei for individual masses A > 257 may not represent the complete set.[11][13]
The general patterns of beta-stability are expected to continue into the region of superheavy elements, though the exact location of the center of the valley of stability is model dependent. It is widely believed that an island of stability exists along the beta stability line for isotopes of elements around copernicium that are stabilized by shell closures in the region; such isotopes would decay primarily through alpha decay or spontaneous fission.[14] Beyond the island of stability, various models that correctly predict many known beta-stable isotopes also predict anomalies in the beta-stability line that are unobserved in any known nuclides, such as the existence of two beta-stable nuclides with the same odd mass number.[11][15] This is a consequence of the fact that a semi-empirical mass formula must consider shell correction and nuclear deformation, which become far more pronounced for heavy nuclides.[15][16]
The beta-stable fully ionized nuclei (with all electrons stripped) are somewhat different. Firstly, if a proton-rich nuclide can only decay by electron capture (because the energy difference between the parent and daughter is less than 1.022 MeV, the amount of decay energy needed for positron emission), then full ionization makes decay impossible. This happens for example for 7Be.[17] Moreover, sometimes the energy difference is such that while β− decay violates conservation of energy for a neutral atom, bound-state β− decay (in which the decay electron remains bound to the daughter in an atomic orbital) is possible for the corresponding bare nucleus. Within the range 2 ≤ A ≤ 270, this means that 163Dy, 193Ir, 205Tl, 215At, and 243Am among beta-stable neutral nuclides cease to be beta-stable as bare nuclides, and are replaced by their daughters 163Ho, 193Pt, 205Pb, 215Rn, and 243Cm.[18]
Beta decay toward minimum mass
[edit]Beta decay generally causes nuclides to decay toward the isobar with the lowest mass (which is often, but not always, the one with highest binding energy) with the same mass number. Those with lower atomic number and higher neutron number than the minimum-mass isobar undergo beta-minus decay, while those with higher atomic number and lower neutron number undergo beta-plus decay or electron capture.
However, there are a few odd-odd nuclides between two beta-stable even-even isobars, that predominantly decay to the higher-mass of the two beta-stable isobars. For example, 40K could either undergo electron capture or positron emission to 40Ar, or undergo beta minus decay to 40Ca: both possible products are beta-stable. The former process would produce the lighter of the two beta-stable isobars, yet the latter is more common.
| Nuclide | Mass | Nuclide | Mass | Nuclide | Mass | ||||
|---|---|---|---|---|---|---|---|---|---|
| Parent | Cl-36 | 35.96830698 | K-40 | 39.96399848 | Ag-108 | 107.905956 | |||
| Minority decay (β+/EC) | 2% to S-36 | 35.96708076 | 10.72% to Ar-40 | 39.9623831225 | 3% to Pd-108 | 107.903892 | |||
| Majority decay (β−) | 98% to Ar-36 | 35.967545106 | 89.28% to Ca-40 | 39.96259098 | 97% to Cd-108 | 107.904184 | |||
| Nuclide | Mass | Nuclide | Mass | Nuclide | Mass | ||||
| Parent | Eu-150m | 149.919747 | Eu-152m1 | 151.9217935 | Am-242 | 242.0595474 | |||
| Minority decay (β+/EC) | 11% to Sm-150 | 149.9172755 | 28% to Sm-152 | 151.9197324 | 17.3% to Pu-242 | 242.0587426 | |||
| Majority decay (β−) | 89% to Gd-150 | 149.918659 | 72% to Gd-152 | 151.9197910 | 82.7% to Cm-242 | 242.0588358 | |||
| Nuclide | Mass | Nuclide | Mass | Nuclide | Mass | ||||
| Parent | Pm-146 | 145.914696 | |||||||
| Minority decay (β−) | 37% to Sm-146 | 145.913041 | |||||||
| Majority decay (β+/EC) | 63% to Nd-146 | 145.9131169 |
- Isotope masses from:
- Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
Notes
[edit]- ^ 48Ca is theoretically capable of beta decay to 48Sc, thus making it not a beta-stable nuclide. However, such a process has never been observed, having a partial half-life greater than 1.1+0.8
−0.6×1021 years, longer than its double beta decay half-life, meaning that double beta decay would usually occur first.[6] - ^ 96Zr is theoretically capable of beta decay to 96Nb, thus making it not a beta-stable nuclide. However, such a process has never been observed, having a partial half-life greater than 2.4×1019 years, longer than its double beta decay half-life, meaning that double beta decay would usually occur first.[7]
- ^ 148Gd was previously thought to be a third beta-stable isobar for mass 148,[5] but according to current mass determinations it has a higher mass than 148Eu and can undergo electron capture. Nevertheless, the mass difference is very small (27.0 keV, even lower than likewise unseen electron capture of 123Te), and only alpha decay has been observed experimentally for 148Gd.
- ^ While the AME2020 atomic mass evaluation gives 222Rn a lower mass than 222Fr,[8] implying beta stability, it is predicted that single beta decay of 222Rn is energetically possible (albeit with very low decay energy),[9] and it falls within the error margin given in AME2020.[8] Hence, current mass determinations cannot decisively determine whether 222Rn is beta-stable or not, though only the alpha decay mode is experimentally known for that nuclide, and the search for beta decay yielded a lower partial half-life limit of 8 years.[9]
- ^ While the AME2020 atomic mass evaluation gives 259Md a lower mass than 259Fm,[8] implying beta stability, the error margin between them is larger than the mass difference.[8] Hence, current mass determinations cannot decisively determine which one of 259Fm and 259Md is beta-stable.
- ^ Discovery of this nuclide is unconfirmed
- ^ There is no known beta-stable isobar for mass 261, although they are known for the surrounding masses 260 and 262. Various models suggest that one of the undiscovered 261Md and 261No should be beta-stable.[10][11]
References
[edit]- ^ Proc. Int. Symposium on Why and How should we investigate Nuclides Far Off the Stability Line", Lysekil, Sweden, August 1966, eds. W. Forsling, C.J. Herrlander and H. Ryde, Stockholm, Almqvist & Wiksell, 1967
- ^ Hansen, P. G. (1979). "Nuclei Far Away from the Line of Beta Stability: Studies by On-Line Mass Separation". Annual Review of Nuclear and Particle Science. 29: 69–119. Bibcode:1979ARNPS..29...69H. doi:10.1146/annurev.ns.29.120179.000441.
- ^ "Interactive Chart of Nuclides (Brookhaven National Laboratory)". Archived from the original on 2020-07-25. Retrieved 2009-06-19.
- ^ Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- ^ a b Tretyak, V.I.; Zdesenko, Yu.G. (2002). "Tables of Double Beta Decay Data — An Update". At. Data Nucl. Data Tables. 80 (1): 83–116. Bibcode:2002ADNDT..80...83T. doi:10.1006/adnd.2001.0873.
- ^ Aunola, M.; Suhonen, J.; Siiskonen, T. (1999). "Shell-model study of the highly forbidden beta decay 48Ca → 48Sc". EPL. 46 (5): 577. Bibcode:1999EL.....46..577A. doi:10.1209/epl/i1999-00301-2.
- ^ Finch, S.W.; Tornow, W. (2016). "Search for the β decay of 96Zr". Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 806: 70–74. Bibcode:2016NIMPA.806...70F. doi:10.1016/j.nima.2015.09.098.
- ^ a b c d Wang, Meng; Huang, W.J.; Kondev, F.G.; Audi, G.; Naimi, S. (2021). "The AME 2020 atomic mass evaluation (II). Tables, graphs and references". Chinese Physics C. 45 (3): 030003. doi:10.1088/1674-1137/abddaf.
- ^ a b Belli, P.; Bernabei, R.; Cappella, C.; Caracciolo, V.; Cerulli, R.; Danevich, F.A.; Di Marco, A.; Incicchitti, A.; Poda, D.V.; Polischuk, O.G.; Tretyak, V.I. (2014). "Investigation of rare nuclear decays with BaF2 crystal scintillator contaminated by radium". European Physical Journal A. 50 (9): 134–143. arXiv:1407.5844. Bibcode:2014EPJA...50..134B. doi:10.1140/epja/i2014-14134-6. S2CID 118513731.
- ^ a b Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ a b c d Koura, H. (2011). Decay modes and a limit of existence of nuclei in the superheavy mass region (PDF). 4th International Conference on the Chemistry and Physics of the Transactinide Elements. Retrieved 18 November 2018.
- ^ Belli, P.; Bernabei, R.; Danevich, F. A.; et al. (2019). "Experimental searches for rare alpha and beta decays". European Physical Journal A. 55 (8): 140–1–140–7. arXiv:1908.11458. Bibcode:2019EPJA...55..140B. doi:10.1140/epja/i2019-12823-2. ISSN 1434-601X. S2CID 201664098.
- ^ Koura, H.; Katakura, J; Tachibana, T; Minato, F (2015). "Chart of the Nuclides". Japan Atomic Energy Agency. Retrieved 30 October 2018.
- ^ Zagrebaev, Valeriy; Karpov, Alexander; Greiner, Walter (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?" (PDF). Journal of Physics. 420 (1): 012001. arXiv:1207.5700. Bibcode:2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001. S2CID 55434734.
- ^ a b Möller, P.; Sierk, A.J.; Ichikawa, T.; Sagawa, H. (2016). "Nuclear ground-state masses and deformations: FRDM(2012)". Atomic Data and Nuclear Data Tables. 109–110: 1–204. arXiv:1508.06294. Bibcode:2016ADNDT.109....1M. doi:10.1016/j.adt.2015.10.002. S2CID 118707897.
- ^ Möller, P. (2016). "The limits of the nuclear chart set by fission and alpha decay" (PDF). EPJ Web of Conferences. 131: 03002:1–8. Bibcode:2016EPJWC.13103002M. doi:10.1051/epjconf/201613103002.
- ^ Bosch, Fritz (1995). "Manipulation of Nuclear Lifetimes in Storage Rings" (PDF). Physica Scripta. T59: 221–229. Bibcode:1995PhST...59..221B. doi:10.1088/0031-8949/1995/t59/030. S2CID 250860726. Archived from the original (PDF) on 2013-12-26.
- ^ Liu, Shuo; Gao, Chao; Xu, Chang (2021). "Investigation of bound state β− decay half-lives of bare atoms". Physical Review C. 104 (2): 024304. doi:10.1103/PhysRevC.104.024304.
External links
[edit]- Decay-Chains https://www-nds.iaea.org/relnsd/NdsEnsdf/masschain.html
- (Russian) Beta-decay stable nuclides up to Z=118
