Wikipedia:Reference desk/Archives/Science/2016 December 11
| Science desk | ||
|---|---|---|
| < December 10 | << Nov | December | Jan >> | December 12 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
December 11
[edit]Is the occlumotor passes in the neck?
[edit]I've read the article here (occulomotor nerve) and I didn't find the relevant information. On the other hand, I've read that cervical trauma can cause damage to this nerve. How can it be? 93.126.88.30 (talk) 00:29, 11 December 2016 (UTC)
- Oculomotor nerve originates in the midbrain and innervates several eye muscles. It does not pass through the neck. What you may be thinking about is uncal herniation, which indeed may affect the oculomotor nerve; but AFAIK this is more typical for head rather than neck trauma. --Dr Dima (talk) 02:29, 11 December 2016 (UTC)
climate warming and record low temperature
[edit]Why would climate warming imply that we'll reach not only average higher temperatures but also new record low temperatures?31.4.138.71 (talk) 15:05, 11 December 2016 (UTC)
- See global weirding. When the polar vortex penetrates further than usual latitudes from around Cuba or Mexico to Southern Canada can get chilly while places like Greenland are warmer than usual because the "real" cold is diluted instead of corralled into a compact, northern, low daylight area. The air is warmed by its surroundings on the way south till it's only about 32°F by the time it gets to the southern tip of Florida (extreme cold for them). Sagittarian Milky Way (talk) 15:58, 11 December 2016 (UTC)
- Test: global weirding. Are you under the impression that format marks can't go inside a link? —Tamfang (talk) 20:44, 11 December 2016 (UTC)
- Test: example.com. Huh. Weird. Sagittarian Milky Way (talk) 20:58, 11 December 2016 (UTC)
- Does warming imply more extreme meteorological events? Extreme event, by definition, are rare, no matter what. It's easy to spot a fake trend here. Maybe global warming does not imply it. --Llaanngg (talk) 17:14, 11 December 2016 (UTC)
- We need to specifically say "formerly extreme events will now occur on a regular basis". Otherwise you get odd statements like "We get a hundred year flood here every decade or so". StuRat (talk) 17:17, 11 December 2016 (UTC)
- I suggest "what was previously a 4σ event will now be a 3σ event". Double sharp (talk) 12:21, 12 December 2016 (UTC)
- There are several ways to look at this. Let's take heavy rainfall as an example. You can choose thresholds (say, X mm/day) and look at changes in their frequency of occurrence, or choose frequency of occurrence (say, 99th percentile) and look at changes in the amount that corresponds to that frequency of occurrence. With more complex events you have to look at a range of characteristics. For example with hurricanes the indications are that warming will cause an increase in the horizontal sizes of storms and probably some increase in intensity, but the number of storms per year may stay about the same or even decline. Shock Brigade Harvester Boris (talk) 19:22, 11 December 2016 (UTC)
- We need to specifically say "formerly extreme events will now occur on a regular basis". Otherwise you get odd statements like "We get a hundred year flood here every decade or so". StuRat (talk) 17:17, 11 December 2016 (UTC)
- bear in mind that the longer you observe a rather random process, the more outliers you will get. It is probably more useful to study the /rate/ at which these special events occur. Greglocock (talk) 01:17, 12 December 2016 (UTC)
Can I say that the definition of "matter" is anything that has an electron?
[edit]According to what I've read on the book "chemistry for dummies" the definition of "matter" is anything that occupies space and also it has mass. But my question is if I can say another definitions, such as: "Matter is anything that has electron" or "Matter is anything anything that has proton."?(Hydrogen doesn't have neutron and that's why I don't chose this definition) 93.126.88.30 (talk) 17:47, 11 December 2016 (UTC)
- No. For example, a neutron, by itself, is also matter. StuRat (talk) 17:49, 11 December 2016 (UTC)
- Right. How I didn't think about it.:). Is electron also considered as matter? 93.126.88.30 (talk) 17:54, 11 December 2016 (UTC)
- Yes, protons, neutrons, and electrons are all normal matter. You might be interested in Matter#Based_on_protons.2C_neutrons_and_electrons. StuRat (talk) 17:59, 11 December 2016 (UTC)
Is hydrophilic molecule a synonym of polar molecule?
[edit]Is hydrophilic molecule a synonym of polar molecule, and hydrophobic is a synonym of non-polar molecule? I'm asking it because in this video (20:30) about the amino acids, she counts the hydrophobic amino acids, and then she counts the polar amino acids. And when I looked for the definition of polar amino acids I reached to this site which claims that the polar amino acids are hydrophylic. 93.126.88.30 (talk) 20:15, 11 December 2016 (UTC)
- It is usually so, but probably not always. Ruslik_Zero 20:33, 11 December 2016 (UTC)
- Carbonate is hydrophilic and non-polar. --OuroborosCobra (talk) 20:57, 11 December 2016 (UTC)
- Some side-chains can have some polarity, but not be polar enough to be hydrophilic. Maybe at certain pH ranges, depending on whether they are charged vs neutral, they can be hydrophilic. Among the amino acid side-chains, those that are completely nonpolar--the alkyl ones--are definitely hydrophobic. I can't recommend reading our Proteinogenic_amino_acid's table of side-chain properties because there is so much uncited data and confusing claims. DMacks (talk) 21:05, 11 December 2016 (UTC)
- Then is there no clear definition to the question what is "polar amino acids"? According to the dictionary: "an α-amino acid in which the functional group attached to the α-carbon (that is, R in RCH(NH3+)COO-) has hydrophilic properties", in other words it means that hydrophilic amino acids are polar amino acids. But you showed me that it's not true. So what is the ideal definition for "polar amino acids"? maybe I can say that the meaning is that there are different charges on each side of the molecule, and normally when we talk about the 20 amino acids, the polar amino acids are also hydrophilic amino acids. Isn't it?93.126.88.30 (talk) 22:09, 11 December 2016 (UTC)
- We didn't exactly "show you that it's not true." My answer, for example, was not in the context of amino acids, which is a small family of compounds. Your original question was about all molecules, and not just amino acids. Generally, hydrophilic amino acids are those with polar side chains. Serine does not have a charged side chain (at biological pH conditions), but is a polar side chain. --OuroborosCobra (talk) 01:51, 12 December 2016 (UTC)
- Then is there no clear definition to the question what is "polar amino acids"? According to the dictionary: "an α-amino acid in which the functional group attached to the α-carbon (that is, R in RCH(NH3+)COO-) has hydrophilic properties", in other words it means that hydrophilic amino acids are polar amino acids. But you showed me that it's not true. So what is the ideal definition for "polar amino acids"? maybe I can say that the meaning is that there are different charges on each side of the molecule, and normally when we talk about the 20 amino acids, the polar amino acids are also hydrophilic amino acids. Isn't it?93.126.88.30 (talk) 22:09, 11 December 2016 (UTC)
- Fluorinated hydrocarbons are usually hydrophobic, despite the strong polarity of the C-F bonds. (Not giving a link to sources because there are really too many: just searching keywords like "polar hydrophobicity fluorinated" in Google or Google Scholar throws up several good ones.) This isn't just polymers like Teflon but also smaller molecules like perfluorooctane, which is actually even more hydrophobic than octane itself. The main reason is the worse packing of fluorocarbon molecules compared to hydrocarbon molecules resulting in weaker van der Waals interactions with water molecules. Double sharp (talk) 12:17, 12 December 2016 (UTC)
- Polar molecules tend to dissolve easily in water, because water itself is polar; but that is about it, and the definitions are quite different. See also Hansen solubility parameter. TigraanClick here to contact me 12:31, 12 December 2016 (UTC)
- It would be interesting to consider this in the context of crown ethers. Many of these are simple ethylene oxide oligomers with complete rotational symmetry between their subunits, so in concept they are nonpolar. (They may be particularly symmetrical when complexed, but of course if the complex is an ion you simply have a salt) Now what gets interesting is that polyethylene glycol, the general term for ethylene oxide oligomers, is well known to be hydrophilic! Something like [1] (is this one of ours?) points out that crown ethers are hydrophobic on the outside and hydrophilic on the inside. So what's the simplest crown ether? Well, ethylene oxide, but that isn't symmetrical. But the next most symmetrical is dioxane --- which is miscible in water! And yet, clearly symmetrical...
- What I haven't looked at yet is conformations of dioxane. It is possible that the compound can become asymmetrical on a molecule by molecule basis, no matter what the formula says. For example, now and then the oxygens might take the end positions in conformation #4 from Cyclohexane conformation, and for that moment the molecule is polar. Wnt (talk) 15:22, 12 December 2016 (UTC)
- In dioxane, the ring is too small to get the oxygens "inside". You need a larger number of atoms in the ring in o..it's stuck being approximately convex. If each "O–C–C–O" unit needs to have the –C–C– linker wrapped "outside" the inner O, you need to have more than 2 O positions. That means 9-crown-3 is the smallest...see lead ref doi:10.1016/S0022-2860(01)00616-0. DMacks (talk) 23:23, 12 December 2016 (UTC)
- @DMacks: Well, our article does say dioxane is miscible with water, despite being clearly uncharged and (approximately) symmetrical. What I was suggesting wasn't that one oxygen was inside and another outside in this case, but that maybe the two could be at opposite ends of, oh, what do you call the eclipsed equivalent of a chair conformation? It's in that figure I mentioned. And I know really large crown ethers are just cyclic PEG and so will be hydrophilic. (I don't know if there are any intermediate-sized crown ethers that are less hydrophilic because the oxygens point in...) Wnt (talk) 01:00, 13 December 2016 (UTC)
- I was commenting on your statement that crown ethers were hydrophobic on the outside, which would suggest not water soluble. Though perhaps your comment wasn't intending to lead to that conclusion? And in contrast dioxane was water soluble, using an argument that "O on the outside" would contribute to miscibility. That makes sense because it allows hydrogen-bonding and local dipolar effects in separate areas even if there is no net molecular dipole. As a further example that removes cyclohexane conformation effects (the one you were contemplating is the "boat conformation"), consider that 1,4-Benzoquinone is slightly soluble in water. DMacks (talk) 03:04, 13 December 2016 (UTC)
- OK, thanks ... true, I've been confusing here. :) And I managed to confuse myself into thinking the boat was something else, thanks for pointing that out! 1,4-Benzoquinone is also an interesting example, yes. There might be more about it here. Wnt (talk) 03:49, 13 December 2016 (UTC)
- I was commenting on your statement that crown ethers were hydrophobic on the outside, which would suggest not water soluble. Though perhaps your comment wasn't intending to lead to that conclusion? And in contrast dioxane was water soluble, using an argument that "O on the outside" would contribute to miscibility. That makes sense because it allows hydrogen-bonding and local dipolar effects in separate areas even if there is no net molecular dipole. As a further example that removes cyclohexane conformation effects (the one you were contemplating is the "boat conformation"), consider that 1,4-Benzoquinone is slightly soluble in water. DMacks (talk) 03:04, 13 December 2016 (UTC)
- @DMacks: Well, our article does say dioxane is miscible with water, despite being clearly uncharged and (approximately) symmetrical. What I was suggesting wasn't that one oxygen was inside and another outside in this case, but that maybe the two could be at opposite ends of, oh, what do you call the eclipsed equivalent of a chair conformation? It's in that figure I mentioned. And I know really large crown ethers are just cyclic PEG and so will be hydrophilic. (I don't know if there are any intermediate-sized crown ethers that are less hydrophilic because the oxygens point in...) Wnt (talk) 01:00, 13 December 2016 (UTC)
- In dioxane, the ring is too small to get the oxygens "inside". You need a larger number of atoms in the ring in o..it's stuck being approximately convex. If each "O–C–C–O" unit needs to have the –C–C– linker wrapped "outside" the inner O, you need to have more than 2 O positions. That means 9-crown-3 is the smallest...see lead ref doi:10.1016/S0022-2860(01)00616-0. DMacks (talk) 23:23, 12 December 2016 (UTC)
Polar air mass meeting tropical air mass
[edit]What happens if a polar air mass meets a tropical air mass? — Preceding unsigned comment added by Uncle dan is home (talk • contribs) 22:54, 11 December 2016 (UTC)
- The difference in temperature and humidity would suggest you'd get a big storm. StuRat (talk) 23:25, 11 December 2016 (UTC)
- More accurately, you'd get a baroclinic zone, along which you may see a low pressure system develop if the passage of a weather disturbance in the upper atmosphere imparts some cyclonic turning to a section of the frontal boundary. If conditions are right, and there are mechanisms to cause quickly rising air over a broad enough area, then a "big storm" may result. – Juliancolton | Talk 23:49, 11 December 2016 (UTC)
- What you've described is simply a front, just like the ones you see on everyday weather maps (in the middle latitudes). Shock Brigade Harvester Boris (talk) 00:01, 12 December 2016 (UTC)
- Not sure what you're trying to distinguish. A front is any boundary between airmasses. – Juliancolton | Talk 03:09, 12 December 2016 (UTC)
- Yup. That was the original question. Should have outdented to clarify that I wasn't replying to you; sorry about that. Shock Brigade Harvester Boris (talk) 03:30, 12 December 2016 (UTC)
- Not sure what you're trying to distinguish. A front is any boundary between airmasses. – Juliancolton | Talk 03:09, 12 December 2016 (UTC)
- What you've described is simply a front, just like the ones you see on everyday weather maps (in the middle latitudes). Shock Brigade Harvester Boris (talk) 00:01, 12 December 2016 (UTC)
- More accurately, you'd get a baroclinic zone, along which you may see a low pressure system develop if the passage of a weather disturbance in the upper atmosphere imparts some cyclonic turning to a section of the frontal boundary. If conditions are right, and there are mechanisms to cause quickly rising air over a broad enough area, then a "big storm" may result. – Juliancolton | Talk 23:49, 11 December 2016 (UTC)
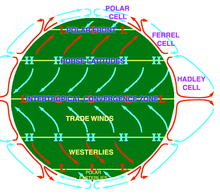
- There is also an interesting question about what would happen to the jet streams. Normally the polar jet stream forms at the intersection of the Polar cell and Ferrel cell, whereas the subtropical jet stream forms at the intersection of the Ferrel cell and Hadley cell. If the Polar cell comes into direct contact with the Hadley cell, what would be the result? It isn't obvious. Another issue is, since the Polar cell and Hadley cell both consist mainly of westward-moving air, would the resulting storm be likely to move westward rather than eastward as usual? Looie496 (talk) 14:35, 12 December 2016 (UTC)
- I searched for those two jet stream names and quickly found [2] - I hope someone here can do much more for us. The quote flummoxed me for a while -- I think the key is that when they say the stream "shifted north" they mean the wind was coming from the north in Pakistan.
Sometimes the jets converge or park above a region. In summer 2010, the polar jet stream shifted north and lingered for more than two months over Eurasia. A stationary high-pressure area developed as a result that disrupted the normal movement of weather systems. This contributed to extreme drought in Russia and devastating floods in Pakistan.
- Wnt (talk) 14:59, 12 December 2016 (UTC)
