Wikipedia:Reference desk/Archives/Science/2012 October 28
| Science desk | ||
|---|---|---|
| < October 27 | << Sep | Oct | Nov >> | October 29 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
October 28
[edit]Generator
[edit]What is the minimal optimal usage for a 30 amp generator to maximize efficiency of fuel? Equidistant from running generator with nothing hooked up and using all 30 amps to run lights in the daytime.68.83.98.40 (talk) 03:03, 28 October 2012 (UTC) Or how many amps does the gen produce just idling?68.83.98.40 (talk) 03:05, 28 October 2012 (UTC)
- If there's nothing hooked up to the generator (as in, open circuit), then it will produce no amps at all.24.23.196.85 (talk) 03:11, 28 October 2012 (UTC)
Yes, that is one extreme in my example. The other extreme is getting "no real use" while producing all 30 amps .68.83.98.40 (talk) 03:16, 28 October 2012 (UTC)
- If you're asking about the point at which the generator will have the highest efficiency, then it depends on the resistance of the circuit, as well as on the characteristics of the diesel that runs the unit. Generally, the higher the circuit's resistance (given the exact same engine and generator unit), the higher the power setting that you'll have to use in order to get the maximum efficiency. Just my non-expert opinion. 24.23.196.85 (talk) 03:25, 28 October 2012 (UTC)
Thanks, anyone know a typical amp output for a 30 amp generator while idling (20 hp?)68.83.98.40 (talk) 03:29, 28 October 2012 (UTC)
- The internal losses in a generator can be modelled as L = k0 + k1I + k2I2 where k0, k1, and k2 are constants particular to the actual generator and I is the output current. Typically, maximum conversion efficiency is attained at around 65% to 80% of full output. Efficiency at no load is obviously zero as 24.23.196.85 already stated, as the amps output is zero but the engine is running and consuming fuel. Fuel consumption at high idle depends on the engine type (recent model, old model, degree of turbo charging) but will be somewhere around 5% of the full load value for generators capable of 30 A per phase at 415 V. Conversly, if the engine is running at high idle, there cannot be any significant electrical output as if there was, the engine would have to slow down or advance the throttle. The correct term for the condition of a genset running without any load ishigh idle as the engine has to be turning at full RPM to get the correct voltage and be ready for any load being instantly switched on. If the engine is run at low speed idle, like a car engine idling, it is called low idle. Keit 124.182.151.138 (talk) 06:05, 28 October 2012 (UTC)
Nanotchnology and solar cells
[edit]How nanosolar cells work ? — Preceding unsigned comment added by41.209.224.61 (talk) 05:21, 28 October 2012 (UTC)
- Nanosolar is the name of a company, see Nanosolar#Technology. They (talk) 05:40, 28 October 2012 (UTC)
what is the development that nanotechnology provides to solar cell? — Preceding unsigned comment added by 41.209.224.61 (talk • contribs)
- Did you click on the link I wrote above? Molecular self-assembly is kind of awesome. They (talk) 09:29, 28 October 2012 (UTC)
i want another resource — Preceding unsigned comment added by41.209.224.61 (talk) 10:43, 28 October 2012 (UTC)
- Well, I don't think we can help you. They (talk) 10:53, 28 October 2012 (UTC)
- why? ...the answer should be from Wikipedia pages — Preceding unsigned comment added by 41.209.224.61 (talk) 11:07, 28 October 2012 (UTC)
- And it is:
| “ | These details involve a semiconductor ink that it claims will enable it to produce solar cells with a basic printing process, rather than using slow and expensive high-vacuum based thin-film deposition processes. The ink is deposited on a flexible substrate (the “paper”), and then nanocomponents in the ink align themselves properly via molecular self-assembly. | ” |
| — Wikipedia editor(s) | ||
- For additional resources dealing with the company, see Nanosolar#References. StuRat (talk) 06:22, 29 October 2012 (UTC)
why is it so hard to find the longitudinal diameter of the hydrogen molecule?
[edit]This is different from the VDW radius or the bond length. I need to calculate the mean free path of hydrogen molecules, not hydrogen gas, and google only turns up the VDW radius of the hydrogen atom or the bond length of the hydrogen molecule, which is annoying. 71.207.151.227(talk) 07:41, 28 October 2012 (UTC)
- Please clarify what you want. Pure hydrogen gas consists of a mixture of monatomic hydrogen (H) and diatomic hydrogen (H2). The proportions of H and H2 are a function of temperature - at room temperature it is virtually all H2, and the proprtion that is H2 decreeases as temperature increases, reaching a 50:50 mix at about 4400 K. You can calculate the proportions by solving the modified arrhenious equations for t → very large, or by using dissociation theory. You can get the constants required in either case from standard tables or from the NIST website.
- Since colliding molecules can have any orientaion, you don't need the longitudinal diameter, unless you introduce a steric factor as well - it will then be equivalent to the collision diameter given in most tables.
- Since hydrogen gas is a mix of H and H2, at high temperatures if you need acuracy you need to calculate for 3 types of collisions: H and H, H and H2, and H2 and H2, at the appropriate concentrations. Wickwack 58.169.248.13 (talk) 12:02, 28 October 2012 (UTC)
- If you know the VDW radius and the bond-length, a diatomic is obviously linear, so the "VDW length" is radius+bond+radius. But depending what you're shooting at it, the scattering cross-section used to calculate the mean free path in an experiment might be some other function rather than simple geometric size (especially one that would need kinetic averaging among various orientations). DMacks (talk) 16:49, 28 October 2012 (UTC)
Elements, other than Hydrogen and Uranium, for nuclear fusion and fission
[edit]It is well-known that hydrogen present in Sun helps in nuclear fusion inside it. Uranium-235 is generally used for nuclear fission on earth by scientists for energy generation. Are there other elements which can be used for nuclear fission and fusion ? Sunny Singh (DAV) (talk) 08:16, 28 October 2012 (UTC)
- See Nuclear fuel cycle, Nuclear power, Fusion power and perhaps Nuclear reprocessing which describes possibilities of main interest in a resonable amount of detail (albeit not in a simple format). The most common proposed alternative to uranium as the fuel source is Thorium-232, note however this for use in a breeder reactor to produce uranium-233 and later 235. You can also design a breeder reactor to use uranium-238. Plutonium-239 may be used together with uranium although this has likely been produced from uranium either intentionally (perhaps for weapons) or as a byproduct as a nuclear reactor. There is also some interest in using the minor actinides byproducts as fuel, our article mentions americium as one currently being tested. When it comes to fusion, deuterium (hydrogen-2) or hydrogen-1 is generally used in most proposals although you may also need boron or lithium as part of the cycle. Nil Einne (talk) 09:23, 28 October 2012 (UTC)
- Here's something interesting: the US has so much neptunium, that they don't know what to do with it all, so they've decided to bury it. Plasmic Physics (talk) 09:45, 28 October 2012 (UTC)
- In principle, most nuclei can be used in fusion, and just that happens in stars. See stellar nucleosynthesis. Similarly, most heavy nuclei can be split. There is a natural maximum of (per nucleon) nuclear binding energy at around Fe56, so "normal" processes will rarely produce heavier elements from lighter ones, and vice versa. There are some fusion- and fission reactions that are more suitable for technological energy generation, and these usually involve very light and very heavy nuclei. --Stephan Schulz (talk) 10:29, 28 October 2012 (UTC)
- Helium-3 is a good material to use for nuclear fusion. 24.23.196.85 (talk) 02:00, 29 October 2012 (UTC)
- In principle, most nuclei can be used in fusion, and just that happens in stars. See stellar nucleosynthesis. Similarly, most heavy nuclei can be split. There is a natural maximum of (per nucleon) nuclear binding energy at around Fe56, so "normal" processes will rarely produce heavier elements from lighter ones, and vice versa. There are some fusion- and fission reactions that are more suitable for technological energy generation, and these usually involve very light and very heavy nuclei. --Stephan Schulz (talk) 10:29, 28 October 2012 (UTC)
Follow up question,
In fission energy emitted by heavy atoms (other than uranium) emit same amount of energy as emitted by uranium, yes or no and why. Does the same case happen with hydrogen in fusion ? Sunny Singh (DAV) (talk) 12:26, 28 October 2012 (UTC)
- No, the amount of energy released is basically different for each nuclear reaction. See the link on nuclear binding energy above - the energy you get from splitting or fusion is the difference in the sum of binding energy before and after the reaction for all involved nucleons. But for most processes, the nuclear binding energy is orders of magnitude greater than for chemical reactions. So any nuclear fuel is relatively very energy-dense. --Stephan Schulz (talk) 14:33, 28 October 2012 (UTC)
- For fusion reactions not taking place inside of a star, fusing elements heavier than hydrogen is very difficult. The reason for this is that the forces required to overcome the electromagnetic repulsion of the nuclei to be fused become pretty prohibitive — and hey, even fusing hydrogen is hard, outside of a star. Even hydrogen bombs don't fuse anything larger than tritium and deuterium. (They use lithium in their fuel, but only because it turns into tritium after being hit with neutrons.) --Mr.98 (talk) 16:04, 28 October 2012 (UTC)
Does the ideal gas law apply to supercritical fluids?
[edit]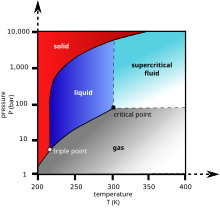
Really basic and important physics here, yet I don't know the answer and our articles don't seem to give it: does the ideal gas law apply to some, all, or no supercritical fluids? What are the boundaries of the region where it applies?
Looking this up in our archive, I found[1] and[2] which expressed doubt it applied; Google delivered[3] which, unless I misinterpret, says it applies to all supercritical fluids. But I have a hard time picturing a supercritical fluid can keep to that law under up to infinite pressure! [4] is a fringy-looking publication which says the critical point is really the boundary of four phases, one of which is a "delta phase" with multiple molecules in association; I think that is saying there is a boundary within supercritical fluids between those which follow ideal gas law and those that don't. In short: please, somebody who really knows their physics should figure this out, and update those two articles accordingly. Thanks. Wnt (talk) 15:42, 28 October 2012 (UTC)
- At the critical point, the specific heat becomes infinite. The specific heat contour lines bend near the CP because of this. Therefore the ideal gas laws cannot apply without significant correction near the critical point. What is happening is most easily seen if you plot specific heat contours on a graph of temperature versus internal energy, along with the saturation (boundary) lines. I haven't figured out how to post diagrams in Ref Desk. If you can tell me the secret of how to do it, I'll give you a diagram or two that will make things clear. As a matter of personal policy, I don't edit WP articles directly, as certain admins have spent a fair amount of effort trying to block/exclude me, and I have no wish to a) waste my time, and b) upset folk. Wickwack124.182.136.192 (talk) 16:09, 28 October 2012 (UTC)
- Well, if you have that kind of trouble, it's probably desirable in general to avoid the tedious scope and copyright issues, etc. (even though they shouldn't apply in this case for your own diagram) of uploading files here or even at Wikimedia Commons - just find any old spot online off the List of photo sharing websites to upload a file and post a link to it here. Wnt (talk) 16:19, 28 October 2012 (UTC)
- On a quick check, I cannot see how to upload to Wikipedia Commons, and think you have to be registered. I post to Ref Desk only using dynamic IP, without a registered username, due to admin actions as mentioned before. I've never used photo sharing sites - I will investigate later today after I get some paying work done if nobody else helps you. Or you might like to do your own plot. It's easily done using the data for several substances listed on the NIST website. Wickwack 120.145.174.229 (talk) 00:56, 29 October 2012 (UTC)
- Well, if you have that kind of trouble, it's probably desirable in general to avoid the tedious scope and copyright issues, etc. (even though they shouldn't apply in this case for your own diagram) of uploading files here or even at Wikimedia Commons - just find any old spot online off the List of photo sharing websites to upload a file and post a link to it here. Wnt (talk) 16:19, 28 October 2012 (UTC)
- In reference to the heat capacity near the critical point, I see we have an article critical exponent, though it is anything but obvious what it all means. [5] seems like an interesting source on the asymptote. All kinds of interesting phenomena, like the hysteresis in density according to temperature (how would life use that? I can barely speculate...) - but to the uninitiated, it gives up its meaning somewhat grudgingly. They give a modified ideal gas law, P = ρRT/(1-bρ) - aρ2, where a is an attraction force constant and b is a repulsion force constant, but I don't know what these are for hydrogen nor why they matter more in one area of the phase diagram (and which?) than another. (They give some gnarlier equations after that...) A clearer answer on where the ideal gas law applies would still help me. Wnt (talk) 04:02, 29 October 2012 (UTC)
- The ideal gas law deviates from real gases at higher pressures, and supercritical fluids are generally under very pressures. Ideal gas law#Deviations from real gases. If you lower the pressure below the critical pressure, you don't have a supercritical fluid. You have an ordinary gas. So, for the range of temperatures and pressures where the ideal gas law is a valid approximation of real behavior, there aren't supercritical fluids. There are the Van der Waals equations, which are also approximations, but are better approximations than the Ideal Gas Law itself. --Jayron32 04:13, 29 October 2012 (UTC)
- It would be useful if the pressure-density curves for some gas (hydrogen would be nice) at a lot of different temperatures posted were someplace handy. Trying the Van der Waals equation for grins, assuming we have a comfy 300 K hydrogen ocean at the pressure of the critical point (12.8 atm = 1300 kPa) on Saturn at I think what the article describes as the bottom of the atmosphere, then from here we see a=0.2476 L2bar/mol2, b=0.02661 L/mol for hydrogen; oxygen is 1.378 L2bar/mol2, 0.03183 L/mol. So for 1 mole of hydrogen:
- (12.8 atm + 1 mol^2*0.2476 L2bar/mol2/V2)(V-(1 mol)(0.02661 L/mol))=(1 mol)(0.08205746 L atm /K mol)(300 K) === (12.8 atm + 0.2444 L2atm/V2)(V-0.02661 L)=24.62 L atm === this cubic seems to work out at V=1.95 for 1 mole of hydrogen.
- (12.8 atm + 1 mol^2*1.378 L2bar/mol2/V2)(V-(1 mol)(0.3183 L/mol))=(1 mol)(0.08205746 L atm/K mol)(300 K) === (12.8 atm + 1.36 L2atm/V2)(V-0.03183 L)=24.62 L atm === this cubic seems to work out at V=1.9 for 1 mole of oxygen.
- Now the outcome here, for these equations, is that there's only a small difference between the hydrogen and the oxygen, i.e. the hydrogen is still about as buoyant (more so, I think) than it is under less compressed conditions - there's no "bubble of gaseous oxygen rising in a sea of liquid hydrogen" implied by these a and b numbers. But I have no idea if the equation applies at all, or if the supercritical hydrogen is far more densely packed - this was just a reality check for what it works out to say. Wnt (talk) 05:55, 29 October 2012 (UTC)
- It would be useful if the pressure-density curves for some gas (hydrogen would be nice) at a lot of different temperatures posted were someplace handy. Trying the Van der Waals equation for grins, assuming we have a comfy 300 K hydrogen ocean at the pressure of the critical point (12.8 atm = 1300 kPa) on Saturn at I think what the article describes as the bottom of the atmosphere, then from here we see a=0.2476 L2bar/mol2, b=0.02661 L/mol for hydrogen; oxygen is 1.378 L2bar/mol2, 0.03183 L/mol. So for 1 mole of hydrogen:
- It's my understanding that the ideal gas law does apply in the supercrit region, so long as you are nor close to the critical point. "Close" in this conext meaning outside the area around the critical point where the specific heat contours bend. However, gasses do indeed depart in a straightforward way from the ideal law at very high pressures. Trust me, it will become very clear to you if you plot or obtain specific heat contours on a temperature vs internal energy chart. Compressibility factors for correcting for the departure are readily available in standard tables, or you can use things like the van der waals formula, which are very inaccurate. Some advanced textbooks have a universal chart as I recall. Wickwack 120.145.174.229(talk) 05:48, 29 October 2012 (UTC)
- Are you referring only to when you are very near both the critical pressure and the critical temperature, or is one or the other sufficient to throw things off? And you're also saying that it holds in the critical region, but not if you go to too high a pressure in it? Wnt (talk) 06:00, 29 October 2012 (UTC)
- Near the CP in both pressure and temperature - just one will not throw things out wrt ideal gas law. It is aproaching the critical point, from any direction, where specific heat becomes infinite, that is the issue. Yes, the ideal gas law holds in the critical region, except in so far as too high a pressure will require a correction. Note that for many substances, the critical point is not at really great pressure. E.g., hydrogen 1.296 MPa; dodecane 1.817 Mpa (~18 atmospheres) Wickwack 120.145.177.211 (talk) 12:43, 29 October 2012 (UTC)
- Are you referring only to when you are very near both the critical pressure and the critical temperature, or is one or the other sufficient to throw things off? And you're also saying that it holds in the critical region, but not if you go to too high a pressure in it? Wnt (talk) 06:00, 29 October 2012 (UTC)
- It's my understanding that the ideal gas law does apply in the supercrit region, so long as you are nor close to the critical point. "Close" in this conext meaning outside the area around the critical point where the specific heat contours bend. However, gasses do indeed depart in a straightforward way from the ideal law at very high pressures. Trust me, it will become very clear to you if you plot or obtain specific heat contours on a temperature vs internal energy chart. Compressibility factors for correcting for the departure are readily available in standard tables, or you can use things like the van der waals formula, which are very inaccurate. Some advanced textbooks have a universal chart as I recall. Wickwack 120.145.174.229(talk) 05:48, 29 October 2012 (UTC)
Laboratory tests
[edit]I'm making a list of legitimate reasons for requesting laboratory tests, and have come up with the following:
On a population level:
- Screening, to prevent the development of avoidable disease.
On the individual level:
- To look for a specific disease predisposition, in a person with a family history of the disease
- To help making or excluding a diagnosis
- To help choose the most appropriate treatment for a disease
- To help predicting the patient's prognosis
- To monitor the patient's disease activity
I'm aware that paternity testing isn't on the list. Other than that, have I left out something important?--NorwegianBlue talk 15:50, 28 October 2012 (UTC)
- You left out monitoring for correct drug dosage. Examples of this is the usage of INR tests to check/adjust the dosage of warfarin, and the the testing of blood cell counts to check/adjust the dosage of chemotherapy for cancer.
- Another reason: During surgery for cancer (lumpectomies), the surgeone makes a judgement call on how much to cut out. Typically, a 2 cm margin of safety is is aimed for. The surgeon sends the excised flesh (hopefully containing the malignant tissue surrounded by the 2 cm margin) to the lab for immediate analysis. While the patient is on the table anethetised and opened up, the lab reports back to the surgeon, essentially saying "you got it all", or "no you didn't - the tumour extends to the edge of the sample". This provides feedback to the surgeon as to his judgement quality, and lets him decide whether or not to cut a bit more out, cognizant of other factors. Wickwack 124.182.136.192 (talk) 15:58, 28 October 2012 (UTC)
- Thanks, I'll add
- To evaluate treatment (or diet) efficacy,
- diet effects (such as monitoring whether gluten is effectively eliminated from the diet) is certainly relevant to the list I'm preparing. Anything else?--NorwegianBlue talk 19:54, 28 October 2012 (UTC)
- Thanks, I'll add
- There are a number of other uses I would think of as illegitimate, but you might not, e.g. testing for recreational drugs, performance enhancing drugs, consumption of banned food/drink/tobacco, identifying the person tested or members of his family as criminal suspects by genetic matching, etc. Wnt(talk) 20:54, 28 October 2012 (UTC)
- I'm not sure whether you'd consider "Monitoring for side effects of treatments" is already covered in your list (under choosing the most effective treatment) but it seems like it should be made explicit. I would also include testing of blood type and HLA types for potential organ donors (I suppose for recipients it would already be covered by "choosing effective treatment". There's also genetic testing for study of population migration/anthropology, and for genealogical curiosity (even when paternity is not in question). And in addition to Wnt's suggestion, CODIS testing of convicted criminals (rather than suspects) might be included, depending on your criteria for "legitimate". - Nunh-huh 01:48, 29 October 2012 (UTC)
- yeah, one more vote for some version of "Monitoring for side effects of treatments", "monitoring for correct drug dosage", etc; want to check for liver enzymes in general, check for rhabdomyolysis due to statins, etc.Gzuckier (talk) 02:03, 29 October 2012 (UTC)
- Without too much consideration as to whether they're already subsumed in one of the listed criteria, I'd think of autopsies (or any pathological testing on the dead, done for the purpose of establishing cause of death, for forensic purposes, the curiosity of the survivors, or assessment of familial risk factors); epidemiologic monitoring (in hospitals; to govern antibiotic usage; to provide monitoring for antibiotic coverage; in animals (to predict flu varieties in order to produce flu vaccine), or any such testing to establish the most common serotypes of viruses or bacteria for vaccine purposes; and testing to determine if preexisting conditions or disease exists for insurance purposes. There's testing for matters of public health ( epidemiology, STD contact tracing, TB drug monitoring (to establish if patients are actually taking their drugs, which is done as much for other's benefit (to avoid the selection for multi drug-resistant strains of TB) as for the "patient's"; and also scientific (rather than clinical) testing (tests which may have eventual clinical significance, but are at present of unproven value). - Nunh-huh 03:14, 29 October 2012 (UTC)
- Some other possibilities:
- Because the patient requested the test. If a patient feels they might have a certain medical condition, and the test isn't risky, then it makes sense to perform the test, if only for their peace of mind.
- To establish a base line. This is important for some repeated tests, like mammograms, as you need something to compare later tests with, to check for any changes. StuRat (talk) 06:17, 29 October 2012 (UTC)
- Is there a non-legitimate reason for requesting a lab test? --TammyMoet (talk) 09:34, 29 October 2012 (UTC)
- "Because the patient requested it" is a reason, if tests are in fact done for that reason. Stu's USA may be different, but doctors are trained to not order a test unless it is their professional opinion that the result will decide which of 2 or more treatment options is to be used. Certainly in Australia they will refuse a patient's unnecessary test - this is because costs are mainly governemnt funded, and the bean counters may penalise a doctor is they think he has cost the govt unnecessary expense. I imagine other countries that have a similar "medicare" or National Health govt funded system (which is most countries apart from 3rd world) will be similar. Wickwack 120.145.177.211 (talk) 11:17, 29 October 2012 (UTC)
- Thanks everyone! @TammyMoet: yes, definitely, there are many. For one thing, for each test that is taken, there is a certain probability that the test will be positive even if the patient is perfectly healthy. If many tests are taken in a non-targeted way, you can be pretty sure that you'll have a false positive among the results. See Ulysses syndrome.This 1954 paper is also a good read, and features a list of what could be considered non-legitimate reasons (see below), although I agree with StuRat that #5, i.e. giving the patient peace of mind, is not without merit. Unless you get a false positive...
- Quoting from the paper: Perhaps it would be a good idea to have a space on every laboratory form in which the doctor had to state exactly why he had ordered a test. I believe if answers were honestly filled in we might get this sort of thing:
- (1) I order this test because if it agrees with my opinion I will believe it, and if it does not I shall disbelieve it.
- (2) I do not understand this test and am uncertain of the normal figure, but it is the fashion to order it.
- (3) When my chief asks if you have done this or that test I like to say yes, so I order as many tests as I can to avoid being caught out.
- (4) I have no clear idea what I am looking for, but in ordering this test I feel in a vague way (like Mr. Micawber) that something might turn up.
- (5) I order this test because I want to convince the patient there is nothing wrong, and I don't think he will believe me without a test.
- --NorwegianBlue talk 11:29, 29 October 2012 (UTC)
- That is a fascinating comment, for the following reasons: Firstly, in my experience, doctors do usually write on the requisistion form in the text box provided something that at least gives a clue as to why they ordered the test. They write abreviated things like "Sus c. rht breast" ie "I suspect carcinoma in right breast", A lot of times they don't need to write anything - the only reason a doctor would order an epstein barr test for example is that he suspects the presence of the eptein barr virus (glandular fever). Secondly, when a quantitative test result comes back from the lab, they usually give three figures like this: Xyzetc 3.0 >3.2 <6.0 ug/ml, which means the Xyzetc concentration was measured at 3.0 micrograms per millilitre, and the normal range is 3.2 to 6.0. A text summary will be at the bottom, saying something like: The Xyzetc was found to be marginally below the normal range for a male of this age group. We suggest you request a so-and-so test to exclude such-and-such disease. This is Australian practice at any rate. A professional would never use reason (4) above. If he got found out he'd be in trouble. Reason (3) will not occur in most countries for the reason I already stated - tests cost money, and someone's counting somewhere. Wickwack 120.145.177.211 (talk) 12:25, 29 October 2012 (UTC)
- Stating on the form what you are looking for could bias the test results, in cases where tests are subjective.
- Also, in the US, another invalid reason for tests is to avoid lawsuits. You can have a group of rare conditions, where the risks of the tests outweigh the benefits, and the risks are not immediately apparent, such as radiation exposure. So, medically, the tests don't make sense, but, legally, the doctor could be sued if the person turns out to have this rare disease, which the test could have found, and he failed to order it. (The test not being medically indicated is not necessarily a sound legal defense, as the law really doesn't much care about science.) StuRat (talk) 18:36, 29 October 2012 (UTC)
- Re avoiding lawsuits, yes, I've heard that tests are ordered in the USA for that reason. In other countries, if a patient wants to sue a doctor, the first thing that happens is that the doctor's practice insurance company reviews that case. If they think the patient just might succeed with a suit, a panel of medical peers is assembled to look at it. Mostly, the peers side with the doctor (surprise surprise), and courts are reluctant to go against the panel opinion. It does keep the number of court cases down.
- Re bias, this is obviously a possibility. However, again in my observation (my wife has quite a history with cancer), if the test is subjective but the result is crucial, doctors tend to requisition from two different labs. If they get opposing or different views back, they either decide which is the right one based on other factors (yep - bias again, possibly NorwegianBlues's item (1)), or they go with the safest option, or they order another sort of test. So, another valid reason to order a test: to confirm or refute a subjective pathologist opinion.
- Wickwack 124.182.21.153 (talk) 01:21, 30 October 2012 (UTC)
- Thanks again, everyone! @Wickwack: This is indeed a valid point, not only when there is a subjective element in the interpretation of the sample. Sample mix-ups occur, and different test methodologies may give discrepant results. --NorwegianBlue talk 07:00, 31 October 2012 (UTC)
how much cropland is lost to circle farming?
[edit]how much cropland is lost to circle farming? Naively, we might assume that 2r*2r is the square equivalent versus pi * rsquared is the circle equivalent. But that would mean 4 rsquared versus 3.141 r squared - i.e. "almost a third" more area is actually available (1.27x more) if they farmed squares instad of farming circles. Is this right though? Because you don't need clearance of a full square around the circle, you can pack the next circle in slightly more closely. I'm asking about this.
http://www.google.com/search?q=circle+farming&tbm=isch
how much cropland is lost to circle famring instad of square farming? --89.132.116.35 (talk) 13:54, 28 October 2012 (UTC)
- You'll want to read the article on Circle packing. I'm not entirely clear on the maths, but it seems that, if the circles are identical, by using hexagonal packing you can cover a maximum of about 0.9069 of the area - that is, a little less than a tenth of the area available is unplanted. - Cucumber Mike (talk) 14:34, 28 October 2012 (UTC) — Precedingunsigned comment added by OsmanRF34 (talk • contribs)
- Though, the question is making a faulty assertion that useful arable land is "lost" because of this farming technique. It makes an overly-simplistic assumption that farm productivity directly corresponds to number of acres planted. In fact, there is a stronger correlation between tons of food produced, andamount of water used. In other words, with enough water, a very small area can yield more crops than a much larger area that uses less water. This is exactly why circular irrigation is executed in practice: it can be a very cost-effective way to put the most amount of water into a useful purpose. You might enjoy reading about agricultural productivity. Agricultural science is incredibly quantitative: if there were a more efficient way to produce more crops at lower cost (keeping in mind that purchasing land and pumping water both have a cost!) - you can bet that technique would be more widespread. If you compare agriculture in different geographic areas - places where the relative costs of land and water are different, or the irrigation needs of the local crops are different - you'll see that some areas don't use circular pivot irrigation. Nimur (talk) 18:51, 28 October 2012 (UTC)
The answers below were added to a dup question at humanities:
- You'll want to read the article on Circle packing. I'm not entirely clear on the maths, but it seems that, if the circles are identical, by using hexagonal packing you can cover a maximum of about 0.9069 of the area - that is, a little less than a tenth of the area available is unplanted. - Cucumber Mike (talk) 14:34, 28 October 2012 (UTC)
- Circle farming is generally associated with center pivot irrigation (CPI). Our article mentions that CPI uses less water and is less labour intensive / requires less maintenance than alternatives for irrigating rectangular areas (RA). As such, the increased cost of irrigating an RA must be juxtaposed with the 10% (hexagonal) / 30% (square) inceased yield of this RA.
- BTW, some further research indicates that the "left-over corners" of circle farms are are, ia, used for storing liquid fertilisers in (quite large) pools which are fed into the CPI system. Also bear in mind that circle farming is used in areas which were useless to agriculture before irrigation became feasible. This was never fertile cropland. --Cookatoo.ergo.ZooM (talk) 20:52, 28 October 2012 (UTC)
- Also note that it would be entirely possible to have overlapping circles so no land goes unwatered. However, this would be more complex, as you'd need to time the rotations so adjacent irrigation systems don't collide (or perhaps cantilever the overlapping portions of each, at different heights, to avoid collision, or just shoot water out the end). You might also want to vary the flow to avoid overwatering the overlap. Another alternative is to use smaller CPI systems to fill in the "holes". I've never seen any of this done, though, so presumably it's more cost efficient just to use those gaps for other purposes, as mentioned above. StuRat (talk) 06:05, 29 October 2012 (UTC)
- Overlapping CPI systems: 32°55′42″N 102°08′48″W / 32.9282°N 102.1467°W (though there's no telling whether they're used at the same time). Filling a gap with a shorter CPI system: 32°46′52″N 102°00′09″W / 32.7810°N 102.0024°W. Both seem to be fairly common. Deor (talk) 13:56, 29 October 2012 (UTC)
- Interesting. I wonder why only half of each large circle appears to be irrigated at the last link, under the Google satellite view. Being kept fallow that year ? StuRat (talk) 18:43, 29 October 2012 (UTC)
- Which link, Stu? μηδείς (talk) 19:11, 29 October 2012 (UTC)
- This one: [6]. StuRat (talk) 19:38, 29 October 2012 (UTC)
- Hm, that's interesting. Odd they would plant four half circles instead of two whole ones. μηδείς (talk) 21:03, 29 October 2012 (UTC)
- Yes, it is. Any farmers here know why they might do that ? Could it be that not using a CPI system for a year risks it rusting and freezing up ?StuRat (talk) 21:07, 29 October 2012 (UTC)
- I'm not a farmer, but it looks to me as if the black specks mainly concentrated near the centers of the green sides of the circles may be cattle (with pens at the circles' centers), which suggests that the fields are for grazing. Maybe planting forage to come up at different times of year in each half?Deor (talk) 22:48, 29 October 2012 (UTC)
- Yes, it is. Any farmers here know why they might do that ? Could it be that not using a CPI system for a year risks it rusting and freezing up ?StuRat (talk) 21:07, 29 October 2012 (UTC)
Using a diesel generator
[edit]We have a generator for the house that operates almost everything (furnace or a/c, stove, lights, water pumps etc, though only one 220-volt draw at a time) when we have power outages. Theoretically, one tankful lasts 8 hours of "normal use". We've never run it for more than 4, so I can't comment on that part. Does it matter how much we operate from it in respect of fuel consumption? Is that what "normal use" refers to? For example, if we only run lights and the water pump, does the generator draw down fuel more slowly than if we also run the electric stove at the same time, or does it draw x amount of fuel just to run, regardless of what is operating from it at the time? Thanks Bielle (talk) 18:07, 28 October 2012 (UTC)
- Yes, more fuel is required to produce more electrical power, because a larger electrical load directly corresponds to more (mechanical) work required to spin the generator. The exact amount of extra fuel consumption is difficult to estimate from first principles; in theory, we could set up a calculation based on conservation of energy, but this doesn't account for all the parasitic effects of friction, or wasted power due to mismatched mechanical or electrical impedance, and so on. This also scales to large power stations: it actually requires a different amount of coal to produce a different amount of electric power; but coal plants aren't easily throttled, so they often run close to peak power production and "burn off" the waste energy. More typically, these plants provide a "base" load, and variable demand loads above the base level are met by activating peaker plants.
- Fortunately, smaller generators can be throttled. I've found numerous charts from various manufacturers who provide an automatic throttle: for example,Honda 3300S Gasoline Generator has an electronic control to slow the engine RPM and reduce fuel consumption when loads are disconnected. This doesn't appear to be a feature on Honda's diesel generator, though. However, even at a fixed RPM, fuel consumption will vary based on the mechanical load on the generator - which, in practice, has at least some correspondance to electrical load. Nimur (talk) 18:35, 28 October 2012 (UTC)
- A internal combustion engine will run most efficiently at full load at the correct operating temperature. Running it at a low and it will will just loose energy through heat loss and friction. Calculate your KVA per gallon and compare that with the cost of mains electricity for the same KVA. Subtract from that the cost of maintenance for you generator. I have heard from people that can maintain their own generator (and cost their time at x$ per hour) and found that it is cheaper to come off line. It boils down to how cheap your fuel is. If you can divert the wast heat (from your generator) into your home then you are quids in.--Aspro (talk) 20:09, 28 October 2012 (UTC)
- Beware overloading the generator and reducing the voltage output - this can kill some things like refrigerators. See brownout (electricity).Wnt (talk) 21:01, 28 October 2012 (UTC)
Hi OP, while much knowledge is displayed above, it is not directly relevant to your question. A diesel gen operates at fixed speed (more or less) and its fuel consumption at that speed is composed of two parts, a fixed quantity per hour, plus an amount that will be roughly proportional to the electrical power you draw.
That is, if you draw the full electrical power it might consume 1.5 gallon per hour, while on no load it might use 1.5 pints an hour (this is estimated for a 2 litre engine at 1800 rpm). Unfortunately the specific figures depend entirely on your engine and generator setup. Generally, you would be better off using it heavily and then switching it off completely, but that is inconvenient for lights etc. Greglocock (talk) 23:35, 28 October 2012 (UTC)
- It is not correct that a genset operates at maxium efficiency at full rated output, for several reasons. Aspro is quite wrong on this. This was covered by another question a few hours ago. The internal losses in a generator can be modelled as L = k0 + k1I + k2I2 where k0, k1, and k2 are constants particular to the actual generator and I is the output current. Typically, maximum conversion efficiency is attained at around 65% to 80% of full output.
- This is because in the generator, there are losses that a constant at the operating RPM (the k0 term), losses proportional to the load current due to magnetic effects (the k1 term) and losses proportional to the square of the load current, due to power lost in a conductor being proportional to the square of current (the k2 term). An internal combustion engine also has losses due to the operating speed (bearing friction, oil pump load, cooling air fan load), proportional to torque (piston ring friction and part of air induction friction loss), plus thermal losses from the cyclinder rising in proprtion to combustion temperature, which is proportional to a power function of load. The thermodynmaic efficiency tends to be a power function of power output in non-computer controlled engines as the required ignition advance (spark in gasoline engines, fuel injection time in diesel engines) can only be correct at a particular power output (centrifugal and vacuum advance [in gasoline engines] are not generally used in genset engines of the sort that would be used to power a home). Engine manufactuers thus design engines so that their best fuel efficiency occurs at the most frequent throttle setting, generally considered to occur around 70% of full throttle. Keit 120.145.29.118 (talk) 00:39, 29 October 2012 (UTC)
- You're mixing up Power band with rpm. Power and efficiency diminishes on the back end of the power curve. You then go on to say what I was indicating, 60 –80% of the rated power. If you were in a helicopter who's power source was an infernal combustion engine and you found yourself sinking on the back-end of the power curve – increasing your rpm would do you little good. On the way down you’re helicopter would guzzle load of fuel, and on impact — you would end up as toast. Full throttle is not max torque. Max rpm is not max torque. Therefore, the OP is best running his generator at max load.--Aspro (talk) 20:17, 29 October 2012 (UTC)
- I haven't confused anything. You have confused the behaviour of gasoline car engines with the behavior of engines designed for stationary service. Car engines have a torque vs RPM curve that peaks at a certain RPM as volumetric efficiency falls as RPM rises, above a certain value, in a carvurettor or throttle body engine. As diesel engine has no air throttle (output is controlled by varying the quantity of fuel injected), the torque vs RPM curve is essentially flat, so max power does occur at max RPM. (Computer control diesel engines may be given a slight drooping torque characteristic for durability reasons). However, a genset engine only operates at constant RPM, and it will be designed to be optimal or near optimal at that RPM. Thermodymanic efficiency does drop a little at full power due to high combustion temperature (high temperature means a greater proportion of heat lost to coolant) and due to the fact that injection timing can only be optimal for only one power setting as I said. I'm not familiar with helicopter engines, but the manufacturer can choose to set the timing to be optimal at wide open throttle at max RPM, rather than at 70% of max power at design RPM as is done with stationary engines. As far as ptrotecting against power loss with increasing RPM, volumetric efficiency can be set to occur at max RPM if desired by intake manifold tuning. Helicopter engines drive what is a dirty great propellor, and would essentailly be forced to be near constant RPM anyway. Keit 124.182.11.117 (talk) 00:58, 30 October 2012 (UTC)
- If you're not using the engine to maximise the electrical power-out-put you are wasting energy in heat. Diesel locomotive divers burn fuel to get up to speed and then coast – as this is most efficient. If you know better, then inform organizations like Amtrak and I'm sure they will reward you handsomely for your breakthrough in finding a way of over turning the laws of thermodynamics. On the obverse: over-fuelling (over running) also increases the need for maintenance and reduces the life of the engine and increase fuel consumption. The users manual will tell you what the max power/efficiency ratio the engine was designed for. An oil- compression engine will also have a governor, so over-speeding does not even come into this – unless it 'diesels' due to broken piston rings. Further more, “stationary engines” and motive engine obey the same laws of thermodynamics - so that don't compute either.
- So, if the OP has (say) a Honda 400 watt (stationary) generator s/he would be better drawing off 400 watts than 200 watts. That IMHO answers the OP's question!
- Your superstition that it should be 70% of 400 watts suggest Honda really selling a 280 watt generator – an accusation that Honda might not like.--Aspro (talk) 02:01, 2 November 2012 (UTC)
- Aspro, you haven't got a clue what you are talking about. Go read a any decent textbook on engines - Charles Fayette Taylor's well known classic The internal-combustion engine in theory and practice, MIT Press, would be a good start. It has loads of formulae you can use. Then, download some performance data for different sorts of engines. While thermodynamics applies to all engines, engines are tailored to the application and behave as I have explained, apart from the fact that diesel engines inherently have a flat torque vs RPM curve as I explained. Railway working is a high mass low friction environment. That's why the locomotive works hard to get up to speed, it has to overcome considerable inertia (F = ma), and once it is up to speed, it only has to overcome friction, which is very low in a train, due to low frontal area for the mass hauled, and due to steel wheels running on steel rails. The very low friction at constant speed in railways is obvious thru making a comparison: A B-double truck can haul up to 80 tonnes or so using typically a 15 litre engine developing 400 KW. A typical railway loco can develop about 3 times as much, but haul loads up to thousands of tonnes. A 40 kW rated generator can be expected to provided 40 kW if the load demands it, but it will run slightly more efficiently at around 30 kW or so, for the reasons stated. A 400 W Honda is quite a different sort of thing to the genset the OP has - he said he has a genset that runs every appliance (tho not all together) - this indicates a 2500 W genset at the very least. In a diesel engine, combustion temperature is proportional to fuel burn. Heat flows from the cylinder gasses (high temp) thru the cylinder walls & head (lower temp) to the coolant. Heat flow to coolant thus rises with power output, as the cylinder walls are in any case at a moderately high but relatively unchanging temperature, the heat flow to the coolant increases to a greater degree than does power output. At power output is increased by increased fuel injection, turbulence also increases, lowering thermal conductivity from gases to cylinder walls & head. The derivation of L = k0 + k1I + k2I2 modelling of a generator is very obvious to an electrical engineer, and the current squared term obviously means effeciency of the generator itself drops above a certain load. Keit 120.145.130.50 (talk) 11:26, 2 November 2012 (UTC)
- you quoted:""the OP has - he said he has a genset that runs every appliance"" then you guess.Lets go back to basics. What generator is is the OP using?--Aspro (talk) 23:17, 2 November 2012 (UTC)
- Aspro, you haven't got a clue what you are talking about. Go read a any decent textbook on engines - Charles Fayette Taylor's well known classic The internal-combustion engine in theory and practice, MIT Press, would be a good start. It has loads of formulae you can use. Then, download some performance data for different sorts of engines. While thermodynamics applies to all engines, engines are tailored to the application and behave as I have explained, apart from the fact that diesel engines inherently have a flat torque vs RPM curve as I explained. Railway working is a high mass low friction environment. That's why the locomotive works hard to get up to speed, it has to overcome considerable inertia (F = ma), and once it is up to speed, it only has to overcome friction, which is very low in a train, due to low frontal area for the mass hauled, and due to steel wheels running on steel rails. The very low friction at constant speed in railways is obvious thru making a comparison: A B-double truck can haul up to 80 tonnes or so using typically a 15 litre engine developing 400 KW. A typical railway loco can develop about 3 times as much, but haul loads up to thousands of tonnes. A 40 kW rated generator can be expected to provided 40 kW if the load demands it, but it will run slightly more efficiently at around 30 kW or so, for the reasons stated. A 400 W Honda is quite a different sort of thing to the genset the OP has - he said he has a genset that runs every appliance (tho not all together) - this indicates a 2500 W genset at the very least. In a diesel engine, combustion temperature is proportional to fuel burn. Heat flows from the cylinder gasses (high temp) thru the cylinder walls & head (lower temp) to the coolant. Heat flow to coolant thus rises with power output, as the cylinder walls are in any case at a moderately high but relatively unchanging temperature, the heat flow to the coolant increases to a greater degree than does power output. At power output is increased by increased fuel injection, turbulence also increases, lowering thermal conductivity from gases to cylinder walls & head. The derivation of L = k0 + k1I + k2I2 modelling of a generator is very obvious to an electrical engineer, and the current squared term obviously means effeciency of the generator itself drops above a certain load. Keit 120.145.130.50 (talk) 11:26, 2 November 2012 (UTC)
- What's an "infernal combustion engine"? 24.23.196.85 (talk) 02:25, 30 October 2012 (UTC)
- One that won't start after you've spent all Saturday working on it. Also, one that won't start when you are about to set off into the city for a job interview. Ratbone 121.215.59.18 (talk) 02:37, 30 October 2012 (UTC)
- You've hit in one!--Aspro (talk) 00:33, 2 November 2012 (UTC)
- One that won't start after you've spent all Saturday working on it. Also, one that won't start when you are about to set off into the city for a job interview. Ratbone 121.215.59.18 (talk) 02:37, 30 October 2012 (UTC)
- I haven't confused anything. You have confused the behaviour of gasoline car engines with the behavior of engines designed for stationary service. Car engines have a torque vs RPM curve that peaks at a certain RPM as volumetric efficiency falls as RPM rises, above a certain value, in a carvurettor or throttle body engine. As diesel engine has no air throttle (output is controlled by varying the quantity of fuel injected), the torque vs RPM curve is essentially flat, so max power does occur at max RPM. (Computer control diesel engines may be given a slight drooping torque characteristic for durability reasons). However, a genset engine only operates at constant RPM, and it will be designed to be optimal or near optimal at that RPM. Thermodymanic efficiency does drop a little at full power due to high combustion temperature (high temperature means a greater proportion of heat lost to coolant) and due to the fact that injection timing can only be optimal for only one power setting as I said. I'm not familiar with helicopter engines, but the manufacturer can choose to set the timing to be optimal at wide open throttle at max RPM, rather than at 70% of max power at design RPM as is done with stationary engines. As far as ptrotecting against power loss with increasing RPM, volumetric efficiency can be set to occur at max RPM if desired by intake manifold tuning. Helicopter engines drive what is a dirty great propellor, and would essentailly be forced to be near constant RPM anyway. Keit 124.182.11.117 (talk) 00:58, 30 October 2012 (UTC)
- You're mixing up Power band with rpm. Power and efficiency diminishes on the back end of the power curve. You then go on to say what I was indicating, 60 –80% of the rated power. If you were in a helicopter who's power source was an infernal combustion engine and you found yourself sinking on the back-end of the power curve – increasing your rpm would do you little good. On the way down you’re helicopter would guzzle load of fuel, and on impact — you would end up as toast. Full throttle is not max torque. Max rpm is not max torque. Therefore, the OP is best running his generator at max load.--Aspro (talk) 20:17, 29 October 2012 (UTC)
How to do a thermometer model like a Galileo Galilei model?
[edit]I mean to build a temprature model of Galileo Galilei. What do I need in order to build this thing in a small form in my house with simple materials and easy to obtain? Thank you for all helpers in instruction. מוטיבציה (talk) 20:32, 28 October 2012 (UTC)
- See Galilean thermometer. My impression is that, like many scientific achievements of the time, it was dependent on glass-blowing skill. But anything you can seal up and attach a weight too should work. Wnt (talk) 20:52, 28 October 2012 (UTC)
- Thank you for help. Until now, I didn't read it properly' but I hope that I will find in it what I need for to do this model of thermometer. One more again, Thank you. מוטיבציה (talk) 14:29, 29 October 2012 (UTC)
- Googling "Galileo thermometer" leads you to many instructions on how to build one yourself. Some of these are easy at-home or intended for school classroom experiments, using simple materials such as tiny jars or bottles and a large vase. The little jars are filled with varying amounts of sand, water or some other ballast for weight. → Michael J Ⓣ Ⓒ Ⓜ 18:50, 29 October 2012 (UTC)
- Hello Michel, I thank you for the information. I hope to find an instruction for the building a model of Galileo exactly, that is to say that I don't need the fake model that published in London about 1990s, see 'Galilean thermometer', I need to build something that was in hands of Galileo, Again, thank you for help. מוטיבציה (talk) 16:31, 30 October 2012 (UTC)
- From our article, it doesn't sound like Galileo actually had anything to do with the Galileo thermometor. It says he did build something called a thermoscope. [7] is listed in the references, and gives some details. Here is an excerpt they give describing the device:
Is this the sort of device you're looking to build? 209.131.76.183 (talk) 12:19, 1 November 2012 (UTC)He took a small glass flask, about as large as a small hen's egg, with a neck about two spans long [perhaps 16 inches] and as fine as a wheat straw, and warmed the flask well in his hands, then turned its mouth upside down into the a vessel placed underneath, in which there was a little water. When he took away the heat of his hands from the flask, the water at once began to rise in the neck, and mounted to more than a span above the level of the water in the vessel. The same Sig. Galileo had then made use of this effect in order to construct an instrument for examining the degrees of heat and cold.
- Yes, it is. Thank you.מוטיבציה (talk) 20:26, 3 November 2012 (UTC)
- From our article, it doesn't sound like Galileo actually had anything to do with the Galileo thermometor. It says he did build something called a thermoscope. [7] is listed in the references, and gives some details. Here is an excerpt they give describing the device:
- Hello Michel, I thank you for the information. I hope to find an instruction for the building a model of Galileo exactly, that is to say that I don't need the fake model that published in London about 1990s, see 'Galilean thermometer', I need to build something that was in hands of Galileo, Again, thank you for help. מוטיבציה (talk) 16:31, 30 October 2012 (UTC)
- Googling "Galileo thermometer" leads you to many instructions on how to build one yourself. Some of these are easy at-home or intended for school classroom experiments, using simple materials such as tiny jars or bottles and a large vase. The little jars are filled with varying amounts of sand, water or some other ballast for weight. → Michael J Ⓣ Ⓒ Ⓜ 18:50, 29 October 2012 (UTC)
- Thank you for help. Until now, I didn't read it properly' but I hope that I will find in it what I need for to do this model of thermometer. One more again, Thank you. מוטיבציה (talk) 14:29, 29 October 2012 (UTC)
