Wikipedia:Reference desk/Archives/Science/2010 September 24
| Science desk | ||
|---|---|---|
| < September 23 | << Aug | September | Oct >> | September 25 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
September 24
[edit]How do autotransformers step up?
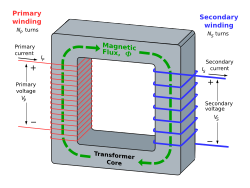
[edit]I'm still trying to wrap my head around this. As far as I can see, an autotransformer is no different to a potential divider or variable resistor (using inductors instead of resistors of course). I can see how voltage could be stepped down (using the potential divider effect), but how is it stepped up? If you inject the primary current halfway up the winding, how is it that the secondary side can be tapped off above the point where the primary current is injected? Surely you'll just get current flow in the top half of the winding, which would act as an inductor in series with the load? Zunaid 08:07, 24 September 2010 (UTC)
- Do you understand how a normal transformer works?
- All winding turns in a single phase transformer has (almost) the same magnetic flux Φ, the induced voltage in each winding turn is proportional to the rate of change of the flux dΦ/dt. If we assume no resistance the induced voltage in each winding turn will be U/Np, U is the applied voltage, Np is the number of turns in the primary winding. The dIp/dt will be adapted to induce the correct dΦ/dt in the core. Any coil on the core will have a induced voltage of dΦ/dt times the number of turns. The fact that voltage is applied to part of the coil does not change this.
- Resistance causes voltage-drop in all winding turns proportional to the current in that winding, flux leakage cause differences in the induced voltage in different turns. The current needed to get the needed flux depends on the permeability and saturation of the magnetic core, eddy currents, currents in other windings and magnetic hysteresis.--Gr8xoz (talk) 08:55, 24 September 2010 (UTC)
- "The fact that voltage is applied to part of the coil does not change this." WHY? If you only apply voltage halfway up the coil (with the bottom point grounded to complete the circuit) it makes logical sense to me that you'd only induce magnetic flux in the half of the coil that is part of the primary circuit. How the heck does the upper half get energised for you tap off a stepped-up secondary voltage? And yes, I should hope I know how a normal transformer works, else I'd be lousy at my job ;) It's just autotransformers that have me quite stumped. Zunaid 10:22, 24 September 2010 (UTC)
- I do not want to offend you but I do not think you really understand how transformers work. It is exactly the same principle. I do not know what step you do not understand so I will try to point out some pitfalls, much of this will probably be obvious to you. As Yyy suggests you can think of an autotransformer as an ordinary transformer with the windings connected in series. In step up configuration the voltage across the secondary winding (high voltage output) is then added to the input voltage and forms a new even higher output voltage. The key thing to understand is that the same magnetic flux goes through every winding-turn on the same core and induces the same voltage in each. The different parts of the coil does not act as independent inductances see Inductive coupling. The magnetic flux in the part of the winding that are connected to the primary circuit goes through all the windings be course of geometry and that the permeability (magnetic conductivity) is much higher in the magnetic (iron) core than in air. The air acts as a (non ideal) insulator in the magnetic circuit.--Gr8xoz (talk) 11:28, 24 September 2010 (UTC)
- I just want to point out that all ”understanding” in this area is relative, nobody understands why the physical laws are as they are. Inductance and magnetic forces can be explained by Relativistic_electromagnetism#The_origin_of_magnetic_forces but why there are electric interaction meditated by photons is to my knowledge unknown. I know that there are some pattern “symmetries” in the theory that suggests it but no explanation. Richard Fyenman talks about this in [1]. I hope I can help the OP (Zunaid) understand this in terms of how to apply known laws of nature but that does not “explain it”.--Gr8xoz (talk) 12:00, 24 September 2010 (UTC)
- I do not want to offend you but I do not think you really understand how transformers work. It is exactly the same principle. I do not know what step you do not understand so I will try to point out some pitfalls, much of this will probably be obvious to you. As Yyy suggests you can think of an autotransformer as an ordinary transformer with the windings connected in series. In step up configuration the voltage across the secondary winding (high voltage output) is then added to the input voltage and forms a new even higher output voltage. The key thing to understand is that the same magnetic flux goes through every winding-turn on the same core and induces the same voltage in each. The different parts of the coil does not act as independent inductances see Inductive coupling. The magnetic flux in the part of the winding that are connected to the primary circuit goes through all the windings be course of geometry and that the permeability (magnetic conductivity) is much higher in the magnetic (iron) core than in air. The air acts as a (non ideal) insulator in the magnetic circuit.--Gr8xoz (talk) 11:28, 24 September 2010 (UTC)
- Autotransformer also could be similar to transformer with connected windings, which can step up. Lower voltage winding part of autotransformer acts both as low voltage winding and as part of high voltage winding. -Yyy (talk) 08:55, 24 September 2010 (UTC)
- .
- ... an attempt to explain in different words.
- As explained above, it doesn't matter whether the coils are separate (as in the ideal transformer on the right), or combined. It is the induced magnetic flux through the core that matters, and this flux extends through the core to energize the secondary coils. The analogy with resistors breaks down because the flux in the core provides the "driving force" for the output, not the applied EMF as in a potential divider. The circuit is not just two inductors in series, in fact it behaves as two separate circuits with a common ground. Dbfirs 12:31, 24 September 2010 (UTC)
- .
- .
- To explain Yyy in other words - Another way of looking at an stepup-autotransformer is a normal isolating transformer with the secondary's low end connected to the primary's high end. If both have the same turn ratio (i.e. the transformer is centre-tapped), then when 100V AC (sin-wave) is applied to the primary another 100V AC is induced with the same phase on the secondary (assuming no loses). The voltages thus add up to the expected 200 V AC. CS Miller (talk) 12:51, 24 September 2010 (UTC)
- Couldn't the questioner model an autotransformer as a 2 winding transformer with one winding of the primary jumpered to one winding of the secondary? Then if he/she understands how a transformer steps up or down voltage, he/she should understand how an autotransformer steps up/down voltage. It is a case of "buck" or "boost." Edison (talk) 03:19, 25 September 2010 (UTC)
- I wrote a decent thank you note to all this morning but lost it when my internet went down. Just wanted to say thanks to everyone especially gr8. Looking at the diagram it is of course blindingly obvious why it should work. The autotransformer article could benefit from having two drawings side by side, one showing a normal transformer and the other an autotransformer. Thanks again! Zunaid 22:16, 26 September 2010 (UTC)
Worst case for Nuclear Waste
[edit]If we assume complete lose of containment of all nuclear waste in the world, all of it is vaporized and evenly distributed in the atmosphere, what would be the consequences.
Of course the rate of cancer would increase but would we be able to live at earth at all?
I am against nuclear power because I think it increases the long-term risk of nuclear war but I think the risks with nuclear waste is often exaggerated so I would find it interesting to know a “worst case”. (The true worst case is of curse that all of it is ingested by humans but that is very unrealistic.)
I know that most of the waste is in ceramic form and will be very insoluble. The relatively low impact of over 500 atmospheric nuclear test indicates low impact but I don't know how to compare it to nuclear waste.
Of course I think we should store the nuclear waste as safe as possible in deep storages any way. --Gr8xoz (talk) 08:11, 24 September 2010 (UTC)
- "(The true worst case is of curse that all of it is ingested by humans but that is very unrealistic.)"
- Hmm, I can't help you with an answer, but I find your original scenario extremely unrealistic, unless governments all over the world did their best to contribute by spreading their nuclear waste into the air. -- Aeluwas (talk) 12:29, 24 September 2010 (UTC)
- If all of the nuclear material in the Earth's crust were "vaporized and evenly distributed in the atmosphere", then we would all die, but, fortunately, loss of containment does not imply dispersal, so I agree with you and Aeluwas that your question is unrealistic. Our article on Radioactive waste gives lots of interesting facts, but doesn't answer your hypothetical question. It does emphasize that a lot of waste comes neither from weapons nor from power generation. Dbfirs 12:51, 24 September 2010 (UTC)
- I know it is unrealistic, the worst I can think of is someone blowing up some of it with nuclear weapons. My goal is to get some baseline to calculate the global consequences of a smaller leakage. Say a leakage of 0.1 % in 100 years, take the radiation dose fore the baseline and multiply by 0.1% and the decay for 100 years and you get some upper limit for the global consequences. I know that it will not spread uniformly and that sedimentation and bioaccumulation will complicate things. Also if we build more nuclear power we can get 1000 times more nuclear waste than now or we can get less than now depending on the type of reactors and our need for energy.--Gr8xoz (talk) 13:00, 24 September 2010 (UTC)
- If all of the nuclear material in the Earth's crust were "vaporized and evenly distributed in the atmosphere", then we would all die, but, fortunately, loss of containment does not imply dispersal, so I agree with you and Aeluwas that your question is unrealistic. Our article on Radioactive waste gives lots of interesting facts, but doesn't answer your hypothetical question. It does emphasize that a lot of waste comes neither from weapons nor from power generation. Dbfirs 12:51, 24 September 2010 (UTC)
- As a matter of examining practical risks, it might be useful to look at the historical events listed at International Nuclear Event Scale as realistic worst-case scenarios. The Chernobyl disaster the Kyshtym disaster (the former involving a nuclear reactor, the latter involving a plant for reprocessing nuclear waste) are the only events in the topmost categories. In the United States, several facilities (including the Hanford Site and the Rocky Flats Plant) are responsible for past or ongoing releases of radioactive materials. There are ongoing lawsuits regarding individuals and populations exposed to contamination, however estimating the deaths which may or may not have resulted is extremely difficult.
- Note that looking at the total and uniform vaporization and atmospheric distribution of all of the world's nuclear waste really isn't anything like a reasonable way to assess risk. Consider that each year, we product roughly sixty million tons of chlorine worldwide; that's enough to administer 20 pounds of the stuff to every man, woman, and child on Earth. Usually when stuff spills – be it nuclear waste or or red wine on a carpet – one observes one or more local hotspots of high contamination, surrounded by regions that are largely unaffected. If vapor is generated, or if waste enters flowing surface or ground water, you will often see a downwind plume of contamination. Instead of uniform distribution, one sees massive overkill caused by very high local concentrations, surrounded by regions of low or negligible contamination. TenOfAllTrades(talk) 13:47, 24 September 2010 (UTC)
- I do not see the similarity with chlorine, if released in the atmosphere it would be to diluted to be toxic and since it is very reactive it will be very short lived. It could of curse be dangerous for a short time locally. Nuclear waste are rather long-lived and have time to spread for thousands of years. The decay of the nuclear waste is independent of its environment while chlorine reacts with the environment and become mostly harmless substances. The nuclear waste loses most of the radioactivity in the beginning and then the long-lived isotopes remains for a long time. Perhaps a release in the ocean is more realistic but I thought it was easier to compare the radioactivity with that of atmospheric testing since we know the average human dose received from that. Background radiation gives 0.15 mSv in 1963.--Gr8xoz (talk) 15:12, 24 September 2010 (UTC)
- If you'll indulge me in a bit of polemic, spraying the air with the waste product of the world's fission power plants isn't a very big deal. It's just uranium. If you really want to worry about us all dying from radiation, consider we've been injecting radioactive coal ash into the airstream for decades and then try to get a good night's sleep ever again. --M@rēino 13:52, 24 September 2010 (UTC)
- It's not "just uranium." It's a load of fission products. They are much more radioactive than the isotopes found in coal. --Mr.98 (talk) 13:57, 24 September 2010 (UTC)
- The scenario you are proposing is just not plausible. The real "worst-case scenarios" are pretty different — things like a terrorist crashing a 747 into a spent fuel site or a massive leak of a specific site occurring. (And the vast volume of nuclear waste is not in ceramic form, but in liquid form.) It's not really comparable to nuclear weapons testing, where a very small volume of material (by comparison) is vaporized and shot into the upper atmosphere (usually) where it can disperse/dilute. The result is a slight up-tick of certain types of cancers, spread amongst a very large population most of the time, only slightly differentiable from the background level. (Whether that is a good enough trade off for the security the nukes were thought to have brought was and is debatable.) By contrast, the worst-case waste scenarios are probably more like Chernobyl (high volumes of very nasty fission products being locally distributed), but even that is a very poor analog. In other words, the worst-case waste scenario is a massive contamination problem for a localized area, not an even distribution/dilution of contamination. --Mr.98 (talk) 13:57, 24 September 2010 (UTC)
- Do you have a source that say that most nuclear waste is in liquid form, I think most of it (by radioactivity) is unprocessed used nuclear fuel. --Gr8xoz (talk) 14:50, 24 September 2010 (UTC)
- Well obviously it depends on whether you are counting it in curies or in volume. I don't have numbers in front of me, but my understanding is that by volume, most of the high-level US waste by volume is military-derived, and most is in liquid form as a result of the reprocessing chemicals (stored at Hanford or Savannah River or other military tank farms). By curies, most is in the form of spent fuel, and is solid. Anyway, it all points to the further complications in this kind of discussion, as "nuclear waste" is actually a pretty broad category of different types of waste. --Mr.98 (talk) 22:51, 24 September 2010 (UTC)
- Do you have a source that say that most nuclear waste is in liquid form, I think most of it (by radioactivity) is unprocessed used nuclear fuel. --Gr8xoz (talk) 14:50, 24 September 2010 (UTC)
- Assume that someone does sneak into the Savannah River Site with a nuclear bomb. I chose this one because it is very large and shallow. It isn't buried under a couple miles of granite. If the nuclear waste was blown into the sky, it would be picked up by the wind. The wind blows from west-to-east. So, it would pretty much follow the Savannah River and settle along the land there. It would probably cause an increase in radioactive health issues in the area around Savannah, Hilton Head, and Beaufort. Then, it will hit the ocean. There is no reason to assume that any significant concentrations of radioactive waste will make it to Europe or Africa. You can easily run a scenario for other sites. For each, some planning went into deciding where to bury the waste. It wasn't just a bunch of monkeys looking for any old hole in the ground. -- kainaw™ 14:04, 24 September 2010 (UTC)
- The weather effects are more complicated. With Savannah River you'd be worried about it being blow north, which is completely plausible on a local scale. The problem with your latter assumption is that Savannah River wasn't chosen because of its characteristics as a waste site, it was chosen for its characteristics as a place to have nuclear reactors. These are not necessarily the same characteristics at all — for example, being next to a river is great for a water-cooled reactor, but lousy from the point of view of burying waste. There are some overlaps, of course: the isolation of the site was chosen in part because of the possibility of meltdown. But none of the current DOE sites were chosen with terrorism in mind, which was a much later consideration. Most were chosen with the idea that the waste would be stored off-site, but that didn't work out. --Mr.98 (talk) 14:26, 24 September 2010 (UTC)
Bird
[edit]Hi. This bird (http://img707.imageshack.us/i/p9240001.jpg/) has entered in my room throught a window, and I have captured it. What is its species? how can I feed it? Thanks. emijrp (talk) 13:16, 24 September 2010 (UTC)
- Two questions: (1) what continent do you live on? (2) are you in the city (implying that this is a pet bird) or the country (implying that this is a wild bird)? --M@rēino 13:54, 24 September 2010 (UTC)
- I'm from Spain, and yes, I live in a city. emijrp (talk) 14:15, 24 September 2010 (UTC)
- i'm no expert, but it looks like someone in your neighbourhood has lost their parrotlet. EverGreg (talk) 14:02, 24 September 2010 (UTC)
- I thought that because I dind't see this bird in the past in my city. I think that it can be an Agapornis. emijrp (talk) 14:15, 24 September 2010 (UTC)
- I have released it. My parents don't want more pets at home (I have another bird). Thanks for your help. emijrp (talk) 14:15, 24 September 2010 (UTC)
Unknown White Powder
[edit]I have got some White powder, which has a bitter taste. On addition of water, it forms a white Suspension (chemistry) which tastes sweet. Effervescence is observed on addition of vinegar to the powder, resulting solution is soapy. What might it be ? —Preceding unsigned comment added by 119.157.63.194 (talk) 14:05, 24 September 2010 (UTC)
- If this isn't homework, perhaps you'd better stop tasting it and adding random other substances to it until you find out (in a proper chemistry lab with proper precautions). If it is homework, we don't do that here, although we will tell you that vinegar in a homework question is just a codeword for its most famous ingredient (mentioned in the article you linked). --Tardis (talk) 14:21, 24 September 2010 (UTC)
- I Just hope that it isn't a rookie in a drug testing lab! -- Q Chris (talk) 15:01, 24 September 2010 (UTC)
- Hey now, chemists used to taste their products all the time. We wouldn't have discovered aspartame or many sweeteners if someone had not accidentally tasted them. But I don't see why something basic would taste sweet. Aspartame is a zwitterion, after all. John Riemann Soong (talk) 15:12, 24 September 2010 (UTC)
- Also maybe its milk powder? If its nonfat dried milk, it wouldn't curdle but maybe bubble upon adding acid. Does the powder react with hydrogen peroxide or baking soda? Does it brown or foam when you add it to a hot frying pan? John Riemann Soong (talk) 15:20, 24 September 2010 (UTC)
- Well, my mother accidentally tasted it, taking for Maida flour. —Preceding unsigned comment added by 119.157.74.205 (talk) 10:42, 25 September 2010 (UTC)
Melting point of water, hydrogen and Oxygen
[edit]Why is it that, even though Hydrogen and Oxygen have melting points in the -300's, that Water, which is comprised of hydrogen and Oxygen, melts at 32 degrees? Buggie111 (talk) 14:07, 24 September 2010 (UTC)
- Because water molecules are asymmetric and so they tend to catch on one another, whereas H2 and O2 are homonuclear diatomic molecules, so they act more like smooth ellipsoids and slide past one another (that is, melt) more easily. --Tardis (talk) 14:15, 24 September 2010 (UTC)
- Is this some weird code for hydrogen bonding? Weird-shaped isobutane is less symmetric than say n-butane, and in fact has a lower BP cuz of its weird shape. John Riemann Soong (talk) 15:16, 24 September 2010 (UTC)
- Yes, it is — I didn't want to assume that the OP would find that discussion useful. Both fish hooks and tippe tops are asymmetric; perhaps I should have said "asymmetric in a way that causes them to catch". --Tardis (talk) 15:25, 24 September 2010 (UTC)
- Is isobutane really less symmetric? Considering just the carbon skeleton, it's got a permanent C3 axis. The only guarantee for n-butane is C2; there may be others (additional C2, one or two mirror planes, a point-inversion) but only in specific conformations. Hard to make a definitive statement about "more" if the things you are comparing aren't the same (operators) and can change. DMacks (talk) 16:16, 24 September 2010 (UTC)
- I really meant to say isopentane. John Riemann Soong (talk) 19:58, 24 September 2010 (UTC)
- Is isobutane really less symmetric? Considering just the carbon skeleton, it's got a permanent C3 axis. The only guarantee for n-butane is C2; there may be others (additional C2, one or two mirror planes, a point-inversion) but only in specific conformations. Hard to make a definitive statement about "more" if the things you are comparing aren't the same (operators) and can change. DMacks (talk) 16:16, 24 September 2010 (UTC)
- I think the OP is also missing a very important distinction. Hydrogen and Oxygen have different properties from Water because water is not a mixture of hydrogen and oxygen, it is a chemical compound containing hydrogen and oxygen atoms. Asking why water has such different properties than hydrogen and/or oxygen is akin to asking why you can't just take steel and rubber and glass and throw it into a pile and expect to drive it down the road. Though cars are made of steel and rubber and glass, it is how they are put together that makes it a car. Likewise, though water is composed of hydrogen and oxygen atoms, it is how the hydrogen and oxygen atoms are put together that determines the properties of water. They are put together differently than you would find in a simple mixture of hydrogen and oxygen. --Jayron32 02:55, 25 September 2010 (UTC)
Brown coating(?) that has ruined aluminum parts
[edit]There are some radiators (earth moving equip) made of aluminum that have turned brown. They are no longer weldable and do not attract magnets. An acid wash (Hydrofluoric acid and Sulfuric acid) had no effect. Lab analysis identified aluminum sulfate. Is this removable or has the metal been altered on a molecular level?--74.2.87.75 (talk) 15:18, 24 September 2010 (UTC)
- Our aluminum sulfate article indicates that it will dissolve in neutral or slightly alkaline water, so an acid wash might have been the wrong choice. The article also says that it decomposes when heated above 580 C. Looie496 (talk) 15:57, 24 September 2010 (UTC)
Aluminum pans in the dishwasher
[edit]I've washed some nice, supposedly-dishwasher-safe, aluminum cake pans in the dishwasher with Cascade (detergent) and now the pans appear to have a sort of uneven white coating on them. It looks as though someone has coated the pans with flour, though the pans remain perfectly smooth to the touch. What chemical reaction occurred? Comet Tuttle (talk) 15:36, 24 September 2010 (UTC)
- No, that's not it - this was indeed presumably the low-phosphate Cascade, but this is a definite uneven change in the color of the pans themselves. The pans are perfectly clean, just discolored. It's not an issue of the low-phosphate dishwashing detergent doing a bad job of cleaning the surfaces. Comet Tuttle (talk) 16:50, 24 September 2010 (UTC)
- I've run into this before with aluminum pans that are cleaned in contact with iron or steel pans. I am 99% certain that the contact between the iron/steel and the aluminum causes the creation of an electrochemical cell, with the highly-reactive aluminum acting as a Sacrificial anode. Electrochemically speaking, Aluminum is a very reactive metal; however it usually forms a very thin and impervious coating of aluminum oxide which prevent further corroding, thus aluminum gives the appearance of being inert. It isn't; its just protected by its own corrosion product. However, if it is in contact with iron in a wet, salty environment (like a dishwasher) you can create corrosion by setting up an electrochemical cell, essentially the iron acts as the cathode and the aluminum acts as the anode, and because of the big difference in electrochemical potential between the metals, this can rapidly corrode the aluminum. I have rendered unusuable some very good aluminum pots and utensils in this way, as they become coated with a fine grey powder which resists being cleaned off, but rubs off onto everything, like fingers and food. Ick. Now, I am careful to avoid putting aluminum into my dishwasher. If you are very careful to avoid contact with any other metal, you could still wash it in a dishwasher, but I have found its not worth the risk. --Jayron32 02:48, 25 September 2010 (UTC)
- even without contact with another metal, the aluminium can still be corroded by the alkaline salts, as the oxide coating is dissolved to form aluminate. That with stuff can spread all around the dishwasher. And anodized aluminium gets its colour stripped off very well too. Graeme Bartlett (talk) 22:00, 25 September 2010 (UTC)
- You've probably got anodized aluminum pans. The dishwasher strips off the nice finish, leaving you with th at white undercoating appearance.The high heat of the dishwasher isn't good fror the coating: "the coating will crack from thermal stress if exposed to temperatures above 80 °C." They are not truely "dishwasher safe" if you want to keep their nice black coating. I made the mistake of running my first nice Calphalon pan through the dishwasher, and got the same result. — The Hand That Feeds You:Bite 13:03, 27 September 2010 (UTC)
- Seriously? A frying pan that must stay below 80 °C? --Sean 15:45, 27 September 2010 (UTC)
What disease would cause someone not to be able to eat corn?
[edit]I met somebody who said that they can't eat corn due to some disease, but I don't remember the name of the disease. Does anyone know what it might be? —Preceding unsigned comment added by A Quest For Knowledge (talk • contribs) 16:14, 24 September 2010 (UTC)
- Sorry, we can't answer medical questions that call for a diagnosis. Just ask the person, perhaps? --Sean 16:18, 24 September 2010 (UTC)
- (edit conflict with TotoBaggins twice) By "corn", do you mean Maize? Our article has a short section about a possible allergy, but says that it's rare, and not well studied: Maize#Allergy. Buddy431 (talk) 16:22, 24 September 2010 (UTC)
- OP is not asking for a diagnosis but to be reminded of it's name. Depend what you mean by 'corn.' If you mean by 'corn' the cereal, than it might have been Coeliac disease.--Aspro (talk) 16:31, 24 September 2010 (UTC)
- Celiac disease is an inability to digest gluten. Corn--maize--does not contain gluten. → ROUX ₪ 16:36, 24 September 2010 (UTC)
- But lots of other corns do. DuncanHill (talk) 16:42, 24 September 2010 (UTC)
- Celiac disease is an inability to digest gluten. Corn--maize--does not contain gluten. → ROUX ₪ 16:36, 24 September 2010 (UTC)
- I don't see this as a request for medical advice, so I don't see any problem in answering it. Unfortunately I don't know the answer, though. It used to be thought that people with diverticulitis should avoid corn, but apparently the usefulness of that has been disproved. Allergies are always a possibility. Looie496 (talk) 16:32, 24 September 2010 (UTC)
- (Edit Conflicts) I don't think this query qualifies as a request for a diagnosis or medical advice. The Querant clearly doesn't imply that he/she thinks he/she might have the disorder, nor is he/she asking on behalf of a third party, he/she is merely seeking information on a physiological topic prompted by a past encounter.
- If the original locutor meant 'corn' in the broad sense of cereal grains, the the Gluten article and the links from it to Gluten sensitivity and Gluten-free diet might be relevant. 87.81.230.195 (talk) 16:33, 24 September 2010 (UTC)
- You might be thinking of Eosinophilic esophagitis, symptomatic of allergic reactions to cow's milk, soy, egg, fish and wheat, but can also include corn and all products derived from corn (sugars, corn syrup,...). PЄTЄRS
JVЄСRUМВА ►TALK 16:51, 24 September 2010 (UTC)
- You might be thinking of Eosinophilic esophagitis, symptomatic of allergic reactions to cow's milk, soy, egg, fish and wheat, but can also include corn and all products derived from corn (sugars, corn syrup,...). PЄTЄRS
- To the original poster: The confusion above is because the word corn means different things in different countries, so it's not 100% clear which "corn" you are asking about. In America, corn always means maize, whereas in England, it means, as noted here, "some local cereal grain, like maybe wheat or barley". To Sean: This is not a medical advice question. It's fine. Comet Tuttle (talk) 16:54, 24 September 2010 (UTC)
- Sorry, I'm an American so yes, I meant Maize. If it helps, this came up in casual conversation as we were about to watch a movie. She said she couldn't eat popcorn because she had some disease. I asked her if she could eat corn on the cobb[2] and she said no. I asked her if she had it all her life and she said no. It might be coeliac disease. I know it wasn't a long name, like "muscular dystrophy" and "adrenoleukodystrophy". A Quest For Knowledge (talk) 17:05, 24 September 2010 (UTC)
- It is not c(o)eliac disease, as corn does not contain gluten/gliadin. → ROUX ₪ 17:10, 24 September 2010 (UTC)
- Sorry, I'm an American so yes, I meant Maize. If it helps, this came up in casual conversation as we were about to watch a movie. She said she couldn't eat popcorn because she had some disease. I asked her if she could eat corn on the cobb[2] and she said no. I asked her if she had it all her life and she said no. It might be coeliac disease. I know it wasn't a long name, like "muscular dystrophy" and "adrenoleukodystrophy". A Quest For Knowledge (talk) 17:05, 24 September 2010 (UTC)
- Hmmm...well, here's some more information I can provide. We've gone to dinner a few times and this was the only time she mentioned anything about any food issues. I know that she can eat cheeseburgers, french fries, chicken quesadillas and pepperoni pizza. Does that help? A Quest For Knowledge (talk) 17:20, 24 September 2010 (UTC)
- You might want to consider upping the restaurants you go to with female companions ;-) --Stephan Schulz (talk) 13:13, 25 September 2010 (UTC)
- Well, it's not all that uncommon to be allergic to corn. You can be allergic to corn but not wheat, or vice versa. I don't know what disease name she would have given you, though, other than "allergy", which you probably would have remembered. --Trovatore (talk) 17:24, 24 September 2010 (UTC)
- (edit conflict)Yes, it confirms that despite what people have said above, it cannot be celiac unless she was ordering gluten-free cheeseburger buns and pizza dough. I'm also intrigued by the fact that she can eat quesadillas--were the tortillas flour- or corn-based? If the tortillas were corn-based, then I strongly suspect she has no such disease and merely dislikes corn or had a poor reaction to corn at one point. Many people, especially dining in restaurants, will claim disease or allergy when in reality they simply do not like a particular ingredient. By doing so, they ensure that people won't attempt to get them to eat it. Corn, however, can be an allergen (so can anything really). That being said, my understanding is that it is not a common allergen, and given the prevalence of corn and corn derivatives in everything in North America, such an allergy would make eating anything other than from-scratch home cooking very difficult. (For example, those cheeseburgers? The buns probably contained corn derivatives in the form of corn syrup, and the processed cheese almost certainly contained maltodextrin, a starch derived from both corn and tapioca). One website claims that when trying to recover from candida one should avoid corn. Then again, one should according to them also avoid anything wheat-based, which would knock out the cheeseburgers at least. Some doctors seem to claim that corn should be avoided for diverticulosis, but apparently there is no support in the literature for that. The only thing that I can find, and is borne out by anecdotal experience with an ex, is corn possibly exacerbating the symptoms of Crohn's disease or IBD. Note I am not a doctor. I am a chef, however, which does mean I have a pretty serious interest in food allergies and intolerances. → ROUX ₪ 17:37, 24 September 2010 (UTC)
- I think you really need to ask her again. Food sensitivities are a very easy thing to get garbled. From here on we would just be guessing. She may be referring to a condition set off by the sensitivity and they are very numerous indeed. --Aspro (talk) 17:43, 24 September 2010 (UTC)
- Well, I'd like to give her the impression that I'm a good listener. ;) Anyway, I don't recall if the tortillas were corn or flour based, but I think I might remember her asking about it. This happened before I knew about her not being able to eat corn, so I didn't think anything of it at the time. As Roux suggests, maybe it's Crohn's disease. A Quest For Knowledge (talk) 18:30, 24 September 2010 (UTC)
- Hmmm...well, here's some more information I can provide. We've gone to dinner a few times and this was the only time she mentioned anything about any food issues. I know that she can eat cheeseburgers, french fries, chicken quesadillas and pepperoni pizza. Does that help? A Quest For Knowledge (talk) 17:20, 24 September 2010 (UTC)
Or diverticulitis, but since you said it was not a long name, maybe colitis or crohns —Preceding unsigned comment added by 165.212.189.187 (talk) 18:59, 24 September 2010 (UTC)
- Sigh. As I said above, there is nothing in the literature (according to meta-sites run by trusted orgs) to suggest that avoiding corn is particularly effective when dealing with diverticulitis. → ROUX ₪ 21:17, 24 September 2010 (UTC)
- Sigh. But she could still be avoiding it because of her diverticulitis, whether or not the literature say it is effective. 109.155.33.219 (talk) 23:26, 24 September 2010 (UTC)
movement of a charged particle in an oppositely charged cloud
[edit]I have a uniformly charged cloud of some radius R of some charge +q, distributed infinitesimally over tiny particles, and a charged particle of -q released at rest on its surface. How do I model its movement? I think it will go straight into the centre and I know that the electric field inside this cloud is kqr/R^3. Acceleration of -q should be -kqr^2/(mR^3). But of course as the charged particle moves closer in, R changes and the electric field changes, so acceleration isn't constant so I can't use any kinematics. Any tips? John Riemann Soong (talk) 19:14, 24 September 2010 (UTC)
Wait this is effectively a harmonic oscillator isn't it? OMG. Velocity isn't zero at the centre. John Riemann Soong (talk) 19:36, 24 September 2010 (UTC)
- Sounds similar to Wikipedia:Reference desk/Archives/Science/2008 November 19#What if we cut a hole through Earth?. That is gravity rather than electrostatics, but same concept and could steal the same basic math ideas from shell theorem. DMacks (talk) 19:40, 24 September 2010 (UTC)
- Electrodynamics inside a plasma is a very complicated field. How accurately do you want to model this? A standard plasma treatment will set up charge, momentum, energy, and higher-order-moment flux equations, and solve them. If you want to consider interactions between the dispersed, positively-charged cloud, and the individual test-charge moving through it, you also need to model viscosity. Bittencourt's "Plasma Physics" textbook walks you through the mathematics, starting with an elementary model and adding extra terms to model the dynamics completely. See also, mathematical descriptions of plasma; most common are the "fluid model" style equations, because for simple cases (like your example), these equations can be solved continuously and analytically (rather than numerically). Nimur (talk) 17:15, 26 September 2010 (UTC)
Washing powders that produce fake dirt
[edit]I remember seeing on TV or hearing on radio long ago that washing powders and some other detergents are deliberately made to make the water they are in look dirty. Could anyone supply some more detail on this, the ingredients or chemistry involved? Thanks 92.15.27.8 (talk) 19:47, 24 September 2010 (UTC)
- I have heard of this one. It is a conspiracy theory. Dolphin (t) 04:07, 25 September 2010 (UTC)
- It also seems a it pointless. Unless there is some weird cultural thing in the UK (or whatever) I would expect most people put the powder and their clothes in the washing machine and turn it on and let it run. They don't periodicly check the water to see how dirty it is. Very often they won't look it at all. If they're evaluating a powder, they're likely to see how clean their clothes are, not how dirty the water looks Nil Einne (talk) 16:55, 25 September 2010 (UTC)
- In the UK, nearly all washing machines are front-loaders with a clear plastic door through which you can see the dirty water and clothes swirling around - often watched by dogs or cats. People also sometimes do hand-washing. 92.29.116.227 (talk) 11:06, 27 September 2010 (UTC)
- Do people in the UK, other then perhaps children actually bother to watch the clothes swirling around so that they will see how dirty the water is? It seems unlikely to me. And even more pointless with frontloaders since you can't open them when in use. Aren't washing machines usually stored in laundry rooms, under countertops, closets or other out of the way places in the UK? Don't front loaders use very little water anyway and considering the internals are not lighted and the tub would usually be silvery can you even really tell that well how dirty the water is? How many people nowdays do enough hand washing and care how dirty the water looks (considering if you are handwashing, it seems the most likely thing you will do is look at what you're washing and see how clean it looks, 'dirty' water if anything just makes it hard to see that and of course there's also the risk that this 'dirty' water is just going to make your clothes dirty) that manufacturers are going to bother to make their washing powders make the water look dirty? Nil Einne (talk) 12:30, 27 September 2010 (UTC)
- You can easily see the water sloshing around, and glance at it from time to time. They are usually in the kitchen. You seem never to have used one. This is another example of someone from across the pond trying to prove that we drive on the right in the UK. 92.15.25.79 (talk) 21:59, 27 September 2010 (UTC)
- Do people in the UK, other then perhaps children actually bother to watch the clothes swirling around so that they will see how dirty the water is? It seems unlikely to me. And even more pointless with frontloaders since you can't open them when in use. Aren't washing machines usually stored in laundry rooms, under countertops, closets or other out of the way places in the UK? Don't front loaders use very little water anyway and considering the internals are not lighted and the tub would usually be silvery can you even really tell that well how dirty the water is? How many people nowdays do enough hand washing and care how dirty the water looks (considering if you are handwashing, it seems the most likely thing you will do is look at what you're washing and see how clean it looks, 'dirty' water if anything just makes it hard to see that and of course there's also the risk that this 'dirty' water is just going to make your clothes dirty) that manufacturers are going to bother to make their washing powders make the water look dirty? Nil Einne (talk) 12:30, 27 September 2010 (UTC)
- In the UK, nearly all washing machines are front-loaders with a clear plastic door through which you can see the dirty water and clothes swirling around - often watched by dogs or cats. People also sometimes do hand-washing. 92.29.116.227 (talk) 11:06, 27 September 2010 (UTC)
- This goes all the way back to someone in sales and marketing selling modern liquid detergents who has observed that the sodium carbonate added to some washing powders make the water look grey. Grey is associated with dirt. Its origins lay in a sales and marketing copy being taken up by the hoi polloi and repeated ad nauseam.--Aspro (talk) 20:23, 25 September 2010 (UTC)
- It also seems a it pointless. Unless there is some weird cultural thing in the UK (or whatever) I would expect most people put the powder and their clothes in the washing machine and turn it on and let it run. They don't periodicly check the water to see how dirty it is. Very often they won't look it at all. If they're evaluating a powder, they're likely to see how clean their clothes are, not how dirty the water looks Nil Einne (talk) 16:55, 25 September 2010 (UTC)
pupil dilation
[edit]Why do the chemicals that dilate people's pupils for an eye exam also cause very poor near vision for a few hours? Googlemeister (talk) 20:09, 24 September 2010 (UTC)
- Tropicamide lists this phenomenon as a side effect, but does not state why. Obviously mydrasis is what the drops cause, but the article does not itself describe the near vision effects. Googlemeister (talk) 20:48, 24 September 2010 (UTC)

- The Ciliary muscle (which is what focuses the lens) is closely associated with the Iris dilator muscle and the Iris sphincter muscle (which are what control pupil aperture size). Chemicals like tropicamide are probably dilating the ciliary muscle, too, so that the lens can not be accommodated to focus on near objects. I'm guessing. WikiDao ☯ (talk) 21:17, 24 September 2010 (UTC)
- That is why I started off with depth of field. If your camera with a prime 50mm lens focusses 30ft to 35ft there is not much perceptible change of image sharpness. Go macro (close up or near vision) and slight changes makes for so-much-change that you need to stop the lens right down in order to accommodate the whole image from front to back... Look at the images in the link from the desk/Miscellaneous: identify that thing [3] The object is only 2 inches across and yet it is all blurred (appeture wide open). Maybe the article ought to dot the i's and cross the t's. Now you have the background articles, do you think that you can expand it without further help?. --Aspro (talk) 21:26, 24 September 2010 (UTC)
- When the aperture (your pupil) is very small, it's like a pinhole camera: not a lot of light comes in, but the place it hits on the sensor (your retina) depends only on which direction it was coming in from. If your pupils are dilated, a bit of light that hits a particular spot might have come from a number of places. (Imagine being very small and sitting on one spot in your retina and looking out the pupil: everything you can see is something that could have hit that point.) The rods and cones in your eye (just like the CCD or film in a camera) have no way to tell which direction light is coming from, only where it hit. So, the larger the pupil, the more blurriness; more light is coming in, but it's not as "organized". Paul (Stansifer) 02:18, 25 September 2010 (UTC)
- Right. And that would be more the case for nearby things than for things far away. And there are probably also the lens issues mentioned above. WikiDao ☯ (talk) 02:39, 25 September 2010 (UTC)
- When the aperture (your pupil) is very small, it's like a pinhole camera: not a lot of light comes in, but the place it hits on the sensor (your retina) depends only on which direction it was coming in from. If your pupils are dilated, a bit of light that hits a particular spot might have come from a number of places. (Imagine being very small and sitting on one spot in your retina and looking out the pupil: everything you can see is something that could have hit that point.) The rods and cones in your eye (just like the CCD or film in a camera) have no way to tell which direction light is coming from, only where it hit. So, the larger the pupil, the more blurriness; more light is coming in, but it's not as "organized". Paul (Stansifer) 02:18, 25 September 2010 (UTC)
- Maybe also some Glare (vision) issues? WikiDao ☯ (talk) 23:51, 24 September 2010 (UTC)
- Lots of good responses but special thanks to Paul, his explanation hit home (for me anyway). hydnjo (talk) 20:40, 25 September 2010 (UTC)
- OK that clears it up, the blurred near vision caused by those chemicals is a result of the pupil dilation itself, and would be unavoidable for such examinations. Googlemeister (talk) 13:26, 27 September 2010 (UTC)
- Note: you can see just fine with a large pupil/aperature. It may cause some blurriness from "glare" (which is basically what Paul is describing), but it is the lack of accommodation of the lens to focus on near objects that is most likely the main issue here. WikiDao ☯ (talk) 15:58, 27 September 2010 (UTC)
- OK that clears it up, the blurred near vision caused by those chemicals is a result of the pupil dilation itself, and would be unavoidable for such examinations. Googlemeister (talk) 13:26, 27 September 2010 (UTC)
- Lots of good responses but special thanks to Paul, his explanation hit home (for me anyway). hydnjo (talk) 20:40, 25 September 2010 (UTC)
How does this work? "low intensity pulsed ultra sound equipment has regrown dentin, pulp, gum, and density of mandible and maxilla bone"
[edit]This is quite a claim! From Low_intensity_pulsed_ultrasound - low intensity pulsed ultra sound equipment has regrown dentin, pulp, gum, and density of mandible and maxilla bone but the article doesn't say a word about the process itself...? The Masked Booby (talk) 21:56, 24 September 2010 (UTC)
- Wow, that article sucks. There is quite an extensive medical literature on the topic, and that article doesn't mention any of it. Let me quote from the abstract of a recent review, PMID 18292369:
- "When evaluated clinically, low-intensity pulsed ultrasound was shown to enhance bone repair (most commonly noted as a decrease in healing time), although variations in patient population hindered a definitive claim to clinical effectiveness. In vitro cellular evaluation and in vivo studies on animal models have revealed an increase in cell proliferation, protein synthesis, collagen synthesis, membrane permeability, integrin expression, and increased cytosolic Ca(2+) levels as well as other increased indicators of bone repair in response to low-intensity pulsed ultrasound exposure. Many of the cellular responses to low-intensity pulsed ultrasound mirror the cellular responses to fluid-induced shear flow, suggesting a link between the two as one potential mechanism of action. The considerable amount of information that has been revealed about the behavior of osteoblasts under low-intensity pulsed ultrasound exposure suggests that the exact mechanism of action is complex.".
- Looie496 (talk) 22:26, 24 September 2010 (UTC)
