Vanadium(III) bromide
| |
| Names | |
|---|---|
| IUPAC name
Vanadium(III) bromide
| |
| Other names
Vanadium tribromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.382 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| VBr3 | |
| Molar mass | 290.654 g/mol |
| Appearance | Green-black solid |
| Density | 4 g/cm3 |
| soluble | |
| Solubility | soluble in THF (forms adduct) |
| +2890.0·10−6 cm3/mol | |
| Structure | |
| octahedral | |
| Related compounds | |
Other anions
|
Vanadium(III) chloride |
Other cations
|
Titanium(III) bromide Molybdenum(III) bromide |
Related compounds
|
Vanadium(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
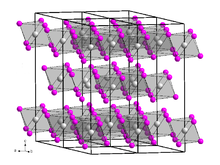
Vanadium(III) bromide, also known as vanadium tribromide, describes the inorganic compounds with the formula VBr3 and its hydrates. The anhydrous material is a green-black solid. In terms of its structure, the compound is polymeric with octahedral vanadium(III) surrounded by six bromide ligands.
Preparation
[edit]VBr3 has been prepared by treatment of vanadium tetrachloride with hydrogen bromide:
- 2 VCl4 + 8 HBr → 2 VBr3 + 8 HCl + Br2
The reaction proceeds via the unstable vanadium(IV) bromide (VBr4), which releases Br2 near room temperature.[1]
It is also possible to prepare vanadium(III) bromide by reacting bromine with vanadium or ferrovanadine:[2]
- 2 V + 3 Br2 → 2 VBr3
- 2 VFe + 6 Br2 → 2 VBr3 + FeBr3
Properties
[edit]Physical
[edit]Vanadium(III) bromide is present in the form of black, leafy, very hygroscopic crystals with a sometimes greenish sheen. It is soluble in water with green color. Its crystal structure is isotypic to that of vanadium(III) chloride with space group R3c (space group no. 167), a = 6.400 Å, c = 18.53 Å. When heated to temperatures of around 500 °C, a violet gas phase is formed, from which, under suitable conditions, red vanadium(IV) bromide can be separated by rapid cooling, which decomposes at −23 °C.[2]
Chemical
[edit]Like vanadium(III) chloride, vanadium(III) bromide forms red-brown soluble complexes with dimethoxyethane and THF, such as mer-VBr3(THF)3.[3]
Aqueous solutions prepared from VBr3 contain the cation trans-[VBr2(H2O)4]+. Evaporation of these solutions give the salt trans-[VBr2(H2O)4]Br.(H2O)2.[4]
Further reading
[edit]- Stebler, A.; Leuenberger, B.; Guedel, H. U. "Synthesis and crystal growth of A3M2X9 (A = Cs, Rb; M = Ti, V, Cr; X = Cl, Br)" Inorganic Syntheses (1989), volume 26, pages 377–85.
References
[edit]- ^ Calderazzo, Fausto; Maichle-Mössmer, Cäcilie; Pampaloni, Guido; Strähle, Joachim (1993). "Low-Temperature Syntheses of Vanadium(III) and Molybdenum(IV) Bromides by Halide Exchange". J. Chem. Soc., Dalton Trans. (5): 655–658. doi:10.1039/DT9930000655.
- ^ a b Brauer, Georg (1975). Handbuch der präparativen anorganischen Chemie Volume 3 (in German). the University of Michigan: Enke. p. 1409. ISBN 978-3-432-87823-2.
- ^ G. W. A. Fowles, G. W. A.; Greene, P. T.; Lester, T. E. "Ether Complexes of Tervalent Titanium and Vanadium" J. Inorg, Nucl. Chem., 1967. Vol. 29. pp. 2365 to 2370.
- ^ Donovan, William F.; Smith, Peter W. (1975). "Crystal and Molecular Structures of Aquahalogenovanadium(III) Complexes. Part I. X-Ray Crystal Structure of trans-Tetrakisaquadibromo-Vanadium(III) Bromide Dihydrate and the Isomorphous Chloro- Compound". Journal of the Chemical Society, Dalton Transactions (10): 894. doi:10.1039/DT9750000894.
