User:Septaholic/sandbox
 Liquid fluorine (F2 at extremely low temperature) | |||||||||||||||||||||
| Fluorine | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | |||||||||||||||||||||
| Allotropes | alpha, beta (see Allotropes of fluorine) | ||||||||||||||||||||
| Appearance | gas: very pale yellow liquid: bright yellow solid: alpha is opaque, beta is transparent | ||||||||||||||||||||
| Standard atomic weight Ar°(F) | |||||||||||||||||||||
| Fluorine in the periodic table | |||||||||||||||||||||
| |||||||||||||||||||||
| Atomic number (Z) | 9 | ||||||||||||||||||||
| Group | group 17 (halogens) | ||||||||||||||||||||
| Period | period 2 | ||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||
| Electron configuration | [He] 2s2 2p5[3] | ||||||||||||||||||||
| Electrons per shell | 2, 7 | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||
| Melting point | (F2) 53.48 K (−219.67 °C, −363.41 °F)[4] | ||||||||||||||||||||
| Boiling point | (F2) 85.03 K (−188.11 °C, −306.60 °F)[4] | ||||||||||||||||||||
| Density (at STP) | 1.696 g/L[5] | ||||||||||||||||||||
| when liquid (at b.p.) | 1.505 g/cm3[6] | ||||||||||||||||||||
| Triple point | 53.48 K, .252 kPa[7] | ||||||||||||||||||||
| Critical point | 144.41 K, 5.1724 MPa[4] | ||||||||||||||||||||
| Heat of vaporization | 6.51 kJ/mol[5] | ||||||||||||||||||||
| Molar heat capacity | Cp: 31 J/(mol·K)[6] (at 21.1 °C) Cv: 23 J/(mol·K)[6] (at 21.1 °C) | ||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | common: −1 | ||||||||||||||||||||
| Electronegativity | Pauling scale: 3.98[3] | ||||||||||||||||||||
| Ionization energies | |||||||||||||||||||||
| Covalent radius | 64 pm[9] | ||||||||||||||||||||
| Van der Waals radius | 135 pm[10] | ||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||
| Crystal structure | cubic | ||||||||||||||||||||
| Thermal conductivity | 0.02591 W/(m⋅K)[11] | ||||||||||||||||||||
| Magnetic ordering | diamagnetic (−1.2×10−4)[12][13] | ||||||||||||||||||||
| CAS Number | 7782-41-4[3] | ||||||||||||||||||||
| History | |||||||||||||||||||||
| Naming | after the mineral fluorite, itself named after Latin fluo (to flow, in smelting) | ||||||||||||||||||||
| Discovery | André-Marie Ampère (1810) | ||||||||||||||||||||
| First isolation | Henri Moissan[3] (June 26, 1886) | ||||||||||||||||||||
| Named by | |||||||||||||||||||||
| Isotopes of fluorine | |||||||||||||||||||||
| |||||||||||||||||||||
Fluorine is a chemical element; it has symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is extremely reactive, as it reacts with all other elements except for the light inert gases.
Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb fluo meaning 'to flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern production. Industrial production of fluorine gas for uranium enrichment, its largest application, began during the Manhattan Project in World War II.
Owing to the expense of refining pure fluorine, most commercial applications use fluorine compounds, with about half of mined fluorite used in steelmaking. The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides, or into cryolite, which plays a key role in aluminium refining. Molecules containing a carbon–fluorine bond often have very high chemical and thermal stability; their major uses are as refrigerants, electrical insulation and cookware, and PTFE (Teflon). Pharmaceuticals such as atorvastatin and fluoxetine contain C−F bonds. The fluoride ion from dissolved fluoride salts inhibits dental cavities, and so finds use in toothpaste and water fluoridation. Global fluorochemical sales amount to more than US$15 billion a year.
Fluorocarbon gases are generally greenhouse gases with global-warming potentials 100 to 23,500 times that of carbon dioxide, and SF6 has the highest global warming potential of any known substance. Organofluorine compounds often persist in the environment due to the strength of the carbon–fluorine bond. Fluorine has no known metabolic role in mammals; a few plants and sea sponges[citation needed] synthesize organofluorine poisons (most often monofluoroacetates) that help deter predation.[15]
Characteristics
[edit]Electron configuration
[edit]Fluorine atoms have nine electrons, one fewer than neon, and electron configuration 1s22s22p5: two electrons in a filled inner shell and seven in an outer shell requiring one more to be filled. The outer electrons are ineffective at nuclear shielding, and experience a high effective nuclear charge of 9 − 2 = 7; this affects the atom's physical properties.[3]
Fluorine's first ionization energy is third-highest among all elements, behind helium and neon,[16] which complicates the removal of electrons from neutral fluorine atoms. It also has a high electron affinity, second only to chlorine,[17] and tends to capture an electron to become isoelectronic with the noble gas neon;[3] it has the highest electronegativity of any reactive element.[18] Fluorine atoms have a small covalent radius of around 60 picometers, similar to those of its period neighbors oxygen and neon.[19][20][note 1]
Reactivity
[edit]| External videos | |
|---|---|

The bond energy of difluorine is much lower than that of either Cl
2 or Br
2 and similar to the easily cleaved peroxide bond; this, along with high electronegativity, accounts for fluorine's easy dissociation, high reactivity, and strong bonds to non-fluorine atoms.[21][22] Conversely, bonds to other atoms are very strong because of fluorine's high electronegativity. Unreactive substances like powdered steel, glass fragments, and asbestos fibers react quickly with cold fluorine gas; wood and water spontaneously combust under a fluorine jet.[5][23]
Reactions of elemental fluorine with metals require varying conditions. Alkali metals cause explosions and alkaline earth metals display vigorous activity in bulk; to prevent passivation from the formation of metal fluoride layers, most other metals such as aluminium and iron must be powdered,[21] and noble metals require pure fluorine gas at 300–450 °C (575–850 °F).[24] Some solid nonmetals (sulfur, phosphorus) react vigorously in liquid fluorine.[25] Hydrogen sulfide[25] and sulfur dioxide[26] combine readily with fluorine, the latter sometimes explosively; sulfuric acid exhibits much less activity, requiring elevated temperatures.[27]
Hydrogen, like some of the alkali metals, reacts explosively with fluorine.[28] Carbon, as lamp black, reacts at room temperature to yield tetrafluoromethane. Graphite combines with fluorine above 400 °C (750 °F) to produce non-stoichiometric carbon monofluoride; higher temperatures generate gaseous fluorocarbons, sometimes with explosions.[29] Carbon dioxide and carbon monoxide react at or just above room temperature,[30] whereas paraffins and other organic chemicals generate strong reactions:[31] even completely substituted haloalkanes such as carbon tetrachloride, normally incombustible, may explode.[32] Although nitrogen trifluoride is stable, nitrogen requires an electric discharge at elevated temperatures for reaction with fluorine to occur, due to the very strong triple bond in elemental nitrogen;[33] ammonia may react explosively.[34][35] Oxygen does not combine with fluorine under ambient conditions, but can be made to react using electric discharge at low temperatures and pressures; the products tend to disintegrate into their constituent elements when heated.[36][37][38] Heavier halogens[39] react readily with fluorine as does the noble gas radon;[40] of the other noble gases, only xenon and krypton react, and only under special conditions.[41] Argon does not react with fluorine gas; however, it does form a compound with fluorine, argon fluorohydride.
Phases
[edit]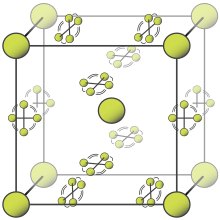
2 molecules that may assume any angle. Other molecules are constrained to planes.

At room temperature, fluorine is a gas of diatomic molecules,[5] pale yellow when pure (sometimes described as yellow-green).[42] It has a characteristic halogen-like pungent and biting odor detectable at 20 ppb.[43] Fluorine condenses into a bright yellow liquid at −188 °C (−306 °F), a transition temperature similar to those of oxygen and nitrogen.[44]
Fluorine has two solid forms, α- and β-fluorine. The latter crystallizes at −220 °C (−364 °F) and is transparent and soft, with the same disordered cubic structure of freshly crystallized solid oxygen,[44][note 2] unlike the orthorhombic systems of other solid halogens.[48][49] Further cooling to −228 °C (−378 °F) induces a phase transition into opaque and hard α-fluorine, which has a monoclinic structure with dense, angled layers of molecules. The transition from β- to α-fluorine is more exothermic than the condensation of fluorine, and can be violent.[48][49][note 3]
Isotopes
[edit]Only one isotope of fluorine occurs naturally in abundance, the stable isotope 19
F.[50] It has a high magnetogyric ratio[note 4] and exceptional sensitivity to magnetic fields; because it is also the only stable isotope, it is used in magnetic resonance imaging.[52] Eighteen radioisotopes with mass numbers from 13 to 31 have been synthesized, of which 18
F is the most stable with a half-life of 109.77 minutes. 18
F is a natural trace radioisotope produced by cosmic ray spallation of atmospheric argon as well as by reaction of protons with natural oxygen: 18O + p → 18F + n.[53] Other radioisotopes have half-lives less than 70 seconds; most decay in less than half a second.[54] The isotopes 17
F and 18
F undergo β+ decay and electron capture, lighter isotopes decay by proton emission, and those heavier than 19
F undergo β− decay (the heaviest ones with delayed neutron emission).[54][55] Two metastable isomers of fluorine are known, 18m
F, with a half-life of 162(7) nanoseconds, and 26m
F, with a half-life of 2.2(1) milliseconds.[56]
Occurrence
[edit]Universe
[edit]| Atomic number |
Element | Relative amount |
|---|---|---|
| 6 | Carbon | 4,800 |
| 7 | Nitrogen | 1,500 |
| 8 | Oxygen | 8,800 |
| 9 | Fluorine | 1 |
| 10 | Neon | 1,400 |
| 11 | Sodium | 24 |
| 12 | Magnesium | 430 |
Among the lighter elements, fluorine's abundance value of 400 ppb (parts per billion) – 24th among elements in the universe – is exceptionally low: other elements from carbon to magnesium are twenty or more times as common.[58] This is because stellar nucleosynthesis processes bypass fluorine, and any fluorine atoms otherwise created have high nuclear cross sections, allowing collisions with hydrogen or helium to generate oxygen or neon respectively.[58][59]
Beyond this transient existence, three explanations have been proposed for the presence of fluorine:[58][60]
- during type II supernovae, bombardment of neon atoms by neutrinos could transmute them to fluorine;
- the solar wind of Wolf–Rayet stars could blow fluorine away from any hydrogen or helium atoms; or
- fluorine is borne out on convection currents arising from fusion in asymptotic giant branch stars.
Earth
[edit]Fluorine is the thirteenth most common element in Earth's crust at 600–700 ppm (parts per million) by mass.[61] Though believed not to occur naturally, elemental fluorine has been shown to be present as an occlusion in antozonite, a variant of fluorite.[62] Most fluorine exists as fluoride-containing minerals. Fluorite, fluorapatite and cryolite are the most industrially significant.[61][63] Fluorite (CaF
2), also known as fluorspar, abundant worldwide, is the main source of fluoride, and hence fluorine. China and Mexico are the major suppliers.[63][64][65][66][67] Fluorapatite (Ca5(PO4)3F), which contains most of the world's fluoride, is an inadvertent source of fluoride as a byproduct of fertilizer production.[63] Cryolite (Na
3AlF
6), used in the production of aluminium, is the most fluorine-rich mineral. Economically viable natural sources of cryolite have been exhausted, and most is now synthesised commercially.[63]
-
Fluorite: Pink globular mass with crystal facets
-
Cryolite: A parallelogram-shaped outline with diatomic molecules arranged in two layers
Other minerals such as topaz contain fluorine. Fluorides, unlike other halides, are insoluble and do not occur in commercially favorable concentrations in saline waters.[63] Trace quantities of organofluorines of uncertain origin have been detected in volcanic eruptions and geothermal springs.[68] The existence of gaseous fluorine in crystals, suggested by the smell of crushed antozonite, is contentious;[69][62] a 2012 study reported the presence of 0.04% F
2 by weight in antozonite, attributing these inclusions to radiation from the presence of tiny amounts of uranium.[62]
History
[edit]Early discoveries
[edit]
In 1529, Georgius Agricola described fluorite as an additive used to lower the melting poin
- ^ "Standard Atomic Weights: Fluorine". CIAAW. 2021.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c d e f Jaccaud et al. 2000, p. 381.
- ^ a b c Haynes 2011, p. 4.121.
- ^ a b c d Jaccaud et al. 2000, p. 382.
- ^ a b c Compressed Gas Association 1999, p. 365.
- ^ "Triple Point | The Elements Handbook at KnowledgeDoor". KnowledgeDoor.
- ^ Dean 1999, p. 4.6.
- ^ Dean 1999, p. 4.35.
- ^ Matsui 2006, p. 257.
- ^ Yaws & Braker 2001, p. 385.
- ^ Mackay, Mackay & Henderson 2002, p. 72.
- ^ Cheng et al. 1999.
- ^ Chisté & Bé 2011.
- ^ Lee et al. 2014.
- ^ Dean 1999, p. 564.
- ^ Lide 2004, pp. 10.137–10.138.
- ^ Moore, Stanitski & Jurs 2010, p. 156.
- ^ Cordero et al. 2008.
- ^ Pyykkö & Atsumi 2009.
- ^ a b Greenwood & Earnshaw 1998, p. 804.
- ^ Macomber 1996, p. 230
- ^ Nelson 1947.
- ^ Lidin, Molochko & Andreeva 2000, pp. 442–455.
- ^ a b Wiberg, Wiberg & Holleman 2001, p. 404.
- ^ Patnaik 2007, p. 472.
- ^ Aigueperse et al. 2000, p. 400.
- ^ Greenwood & Earnshaw 1998, pp. 76, 804.
- ^ Kuriakose & Margrave 1965.
- ^ Hasegawa et al. 2007.
- ^ Lagow 1970, pp. 64–78.
- ^ Lidin, Molochko & Andreeva 2000, p. 252.
- ^ Tanner Industries 2011.
- ^ Morrow, Perry & Cohen 1959.
- ^ Emeléus & Sharpe 1974, p. 111.
- ^ Wiberg, Wiberg & Holleman 2001, p. 457.
- ^ Brantley 1949, p. 26.
- ^ Jaccaud et al. 2000, p. 383.
- ^ Pitzer 1975.
- ^ Khriachtchev et al. 2000.
- ^ Burdon, Emson & Edwards 1987.
- ^ Lide 2004, p. 4.12.
- ^ a b Dean 1999, p. 523.
- ^ Pauling, Keaveny & Robinson 1970.
- ^ Bürgi 2000.
- ^ Müller 2009.
- ^ a b Young 1975, p. 10.
- ^ a b Barrett, Meyer & Wasserman 1967.
- ^ National Nuclear Data Center & NuDat 2.1, Fluorine-19.
- ^ Vigoureux 1961.
- ^ Meusinger, Chippendale & Fairhurst 2012, pp. 752, 754.
- ^ SCOPE 50 - Radioecology after Chernobyl Archived 2014-05-13 at the Wayback Machine, the Scientific Committee on Problems of the Environment (SCOPE), 1993. See table 1.9 in Section 1.4.5.2.
- ^ a b National Nuclear Data Center & NuDat 2.1.
- ^ NUBASE 2016, pp. 030001-23–030001-27.
- ^ NUBASE 2016, pp. 030001–24.
- ^ Cameron 1973.
- ^ a b c Croswell 2003.
- ^ Clayton 2003, pp. 101–104.
- ^ Renda et al. 2004.
- ^ a b Jaccaud et al. 2000, p. 384.
- ^ a b c Schmedt, Mangstl & Kraus 2012.
- ^ a b c d e Greenwood & Earnshaw 1998, p. 795.
- ^ Cite error: The named reference
KGS fluorite terminologywas invoked but never defined (see the help page). - ^ Villalba, Ayres & Schroder 2008.
- ^ Kelly & Miller 2005.
- ^ Lusty et al. 2008.
- ^ Gribble 2002.
- ^ Richter, Hahn & Fuchs 2001, p. 3.
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).





