User:Fama Clamosa/Hand/Evolution
- Below are my attempts to describe the evolution of the human hand. A disordered WIP. Fama Clamosa (talk)

| “ | 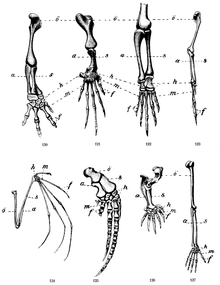 When appendages acquire some form of articulation and muscular autonomous control or become integrated under the control of a central neurological system and permit specific adaptive behaviors coordinated with vision or other sources of relevant information, they are called limbs. Although there are still some gaps in the fossil record, the palaeontology of fishes and early tetrapods allows us to trace back the evolutionary transition from fish to air-breathing, four-legged land vertebrates some 370 millions years ago, when fins evolved into limbs. The evolution of the primate hand, with its characteristic power and precision grips, has been the object of intense scrutiny both neuro-anatomic and functional. Tree-dwelling primates feeding on insects, leaves, fruits and nuts while keeping their balance on limbs or hanging from branches, in addition to grooming, courting, fighting and nurturing, had their hands full, so to speak. Arboreal ecology implies a range of constraints which have molded the early physical and cognitive abilities of primates. Many of these evolved competencies have been conserved in most monkeys and apes, sometimes as the very basis of their existence, sometimes simply making possible alternative ways of life for moving, resting, or hunting. In humans, environmental circumstances can rather easily revive vestigial capacities that enable survival strategies exploiting the resources of trees for escaping danger and providing nutrition. A hardwired grip-reflex is well documented in human neonates and even in the absence of any visible support an adult who trips or loses his/her balance tends to spontaneously produce a grasping gesture. |
” |
| — Bouissac 2004, Gestures in Evolutionary Perspective[1] | ||
| “ | The skeleton of the human hand has its evolutionary origin in the anterior fin of primitive fishes following a reduction process. Starting with 20 to 30 elements, the number of bones reduces to eight. This does not follow a continuous pattern but is influenced by environmental factors. This becomes particularly evident when considering the development of the opposition of the thumb. The muscles of the hand evolve from dorsal and palmar compact plates which are divided into two layers. The differentiation process follows the development of the fingers. The development of the individual hand is controlled by a group of homeobox genes. Comparable genes are found in many different species. The more recent evolution of the hand can be understood as the expression of the development of the brain. Therefore, the hand is a direct tool of our consciousness. It is a main source of differentiated tactile sensations as well as a precise working organ. Gestures, finally, are direct expressions of our personality. | ” |
| — Evolution of the hand, Putz & Tuppek 1999 | ||
| “ |  The fossil record indicates that adaptation for throwing and clubbing began to influence hand structure at or very near the origin of the hominid lineage and continued for millions of years thereafter. During this prolonged period of evolution, the hand underwent a profound remodelling that increasingly adapted it for grasping spheroids in a manner that allows precise control of release and for gripping clubhandles with strength sufficient to withstand a violent impact. Two unique human handgrips were thereby produced. Called the ‘power’ and ‘precision’ grips by Napier (1956) who identified and described them, they can also be referred to as clubbing and throwing grips on the basis of their evolutionary origins.
|
” |
| — Young 2003[2] | ||

pterosaur, bat, and bird
| “ | Symbolic behaviour among humans and non-human primates incorporates the hands, and in human ancestors opportunities to use the hand for this purpose must have increased with the evolution of habitual bipedal posture and locomotion. In tracing the evolution of human symbolic behaviour it is therefore important to trace the origins of human bipedality, and to explore the progressive changes in hominid hand structure and functions that may have affected the use of the hands in communication.
|
” |
| — Evolution of the hand and bipedality, Marzke[3] | ||
| “ | By about 2.6 million years ago, some hominins were making and using simple stone artifacts in eastern Africa. [...] Because the earliest stone artifacts were of such simple construction and because chimpanzees, orangutans, and capuchin monkeys today can employ stones, stems, vines, and sticks to extract nutritious morsels from protective covers, one need not expect that early hominin toolmakers displayed modern hand structure and exquisite motor control.
|
” |
| — Human Evolution, Encyclopædia Britannica[4] | ||
| “ | [...] a majority of primitive features most likely present in the Pan-Homo last common ancestor are retained in the hands of Australopithecus, Paranthropus/early Homo, and Homo floresiensis. This evidence suggests that further derived changes to the hands of other hominins such as modern humans and Neandertals did not evolve until after 2.5 Ma and possibly even later than 1.5 Ma, which is currently the earliest evidence of Acheulian technology. The derived hands of modern humans and Neandertals may indicate a morphological commitment to tool-related manipulative behaviors beyond that observed in other hominins, including those (e.g. H. floresiensis) which may be descended from earlier tool-making species. | ” |
| — The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo., Tocheri et al. 2008[5] | ||
Phylogenetic studies suggest that a primitive autonomization of the first CMC joint occurred in dinosaurs some 365 million years ago; that a real differentiation appeared approximately 70 million years ago in early primates; and that the shape of the human thumb CMC finally appears about 5 million years ago. This evolutionary process has resulted in the human CMC joint being positioned at 80° of pronation, 40° of abduction, and 50° of flexion in relation to an axis passing through the 2nd and 3rd CMC joints. [6]
Tetrapods
[edit]- Tetrapod limbs have five segments: propodium, epipodium, mesopodium, metapodium, and phalanges. The manus and pes are composed of the last three of these.
| General name | Forelimb | Hind limb |
|---|---|---|
| Propodium | humerus | femur |
| Epipodium | radius, ulna | tibia, fibula |
| Manus | Pes | |
| Mesopodium | carpals | tarsals |
| proximal series | radiale intermedium ulnare |
tibiale intermedium fibulare |
| medial series | centralia (4) | centralia (4) |
| distal series | carpalia (5) | tarsalia (5) |
| Metapodium | metacarpals (5) | metatarsals (5) |
| Phalanges | phalanges (5) | phalanges (5) |
Mammals
[edit]What can be more curious than that the hand of man, formed for grasping, that of a mole for digging, the leg of a horse, the paddle of the porpoise and the wing of the bat, should all be constructed on the same pattern and should include similar bones and in the same relative positions.
Charles Darwin, On the Origin of Species
All mammals limbs are based on the pentadactyl limb as an versatile template. [9] In most mammals the primary function of the forelimbs is locomotion, but in others, including primates, cats, and bears, speed and stamina have been sacrificed for an increased range of motion and a wider range of uses -- in turn, providing increased manual dexterity.
In ungulates, hoofed mammals, the forelimb is optimized for speed and endurance by a combination of length of stride and rapid step -- the proximal forelimb segments are short with large muscles, while the distal segments are elongated with less musculature. In two of the major groups of ungulates --Perissodactyla and Artiodactyla-- what remain of the "hands" --metacarpal and phalangeal bones-- are elongated to the extent that they serve little use beyond locomotion. [10]
The terminal phalanges of Chimpanzees are disproportionately small with little apical tufting. [11]
Ungulates
[edit]
In the horse, an example of an odd-toed ungulate Perissodactyla, the forelimb has a single third metacarpal bone equipped with a large metacarpophalangeal joint (fetlock) featuring two posterior sesamoid bones (like in the human thumb). The large proximal and intermediate phalanges end in a specialized terminal phalanges surrounded by the hoof -- a thick derivative of the claw. Likewise, the giraffe, an Artiodactyla and the largest even-toed ungulate, has large terminal phalanges and fused metacarpal bones able to absorb the stress from running.
In even-toed ungulates whose habitats do not require high-speed running over hard terrains, other arrangements of the digits are common. For example, in the Bush Pig (Potamochoerus porcus) the third and fourth toes are weight-bearing, but their non-weight-bearing second and fifth toes are positioned more posteriorly.
Hippopotamus -- four weight-bearing metacarpals. [12]
Cats
[edit]Among the cats designed for speed and strength rather than stamina. short limbs, more muscles distally with greater ranges of motion in the wrist than ungulates. costly limb anatomy. more varied agility -- swat prey, climbing, grooming [13]
Plantigrade
[edit]The plantigrade sloth bear, an insectivorous carnivore, has distal forelimbs resembling feet with carpals arranged similar to the tarsal bones in human feet. However, unlike most mammals, the sloth bear fifth metacarpal is the longest and resembles the human fifth metatarsal. [14]

In the Red Panda, the forelimb digits are also oriented forward but resemble human hands more than the sloth bear. ... feeding behaviour, bamboo. Large radial sesamoid bone reminiscent of opposable thumb
Meerkat, vestigial first digits, MC 2-4 long claws
Sloth, order Pilosa, hang upside-down from branches, highly specialized third and fourth digits (unable to walk on the ground, drags body with claws), short and squat proximal phalanges with much longer terminal phalanges, vestigial second and fifth metacarpals, palm extends to distal IP joint. [15]
Primates
[edit]
Potto
[edit]
Lorids have reduced index fingers. In species such as Perodicticus potto and angwantibo, the index fingers are vestigial, but these arboreal, nocturnal primates can still grip branches with their opposable thumbs. [16]
The third and fourth digits are connected to each other by a slight skin fold, as are the proximal third of toes 3–5. [17]
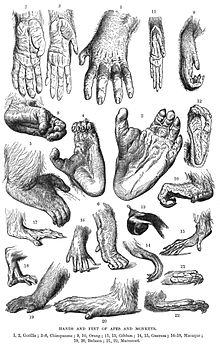
Hominoidae
[edit]
Hylobatidae/Gibbons
[edit]
Asian apes are highly suspensory and have highly mobile ball-and-socket midcarpal joints poorly adapted for weight-bearing. Their hands show many hand features directly related to their locomotor repertoires that are likely uniquely derived yet independently acquired within Pongoidae and Hylobatidae. In orangutan the lunate is expanded radioulnarly, the triqutrum is reduced, the pisisform isprojecting distally, the joint between the trapezium and second metacarpal is expanded palmarly, and the phalanges are highly curved. In hylobatids, the thumb CMC is similar to a ball-and-socket joint.[18]
| “ | Orangutan fingers possess a double-locking mechanism (Napier, '60), and a slight ulnad shift in the axis of the hand which results in lengthened phalanges of ray IV. Hylobatid apes are more like orangutans in their finger morphology than any of the other Hominoidea, but exhibit unique features of their own. These include elongate phalanges of fingers II-V. Human metacarpals II-V form two sets composed of II-III, and IV-V. The heads of both metacarpals II and III are characterized by axial torsion. This reflects the enhanced manipulatory role of the third finger in humans. Human distal phalanges are unique in the development of pronounced apical tufts. | ” |
| — Comparative and functional morphology of hominoid fingers, Randall L. Susman[19] | ||
Siamang
[edit]- Hylobates lar : As is well known, gibbons possess a ball and socket joint at the base of the thumb metacarpal, with the convex (male) surface being located on the trapezium. There are reasonable grounds for assuming that this is a secondary specialization and the joint surface on the first metacarpal may show suggestive indications of derivation from the sellar shape typical of other hominoids. Indeed, as noted above, some specimens of Pongo pygmaeus show a significant approach towards the hylobatid form. As in the pongids described, an anterior oblique ligament is present and a strong posterior oblique ligament radiates from a bony dorsal tubercle on the trapezium.
In one species of gibbon, the large Symphalangus syndactylus ("joined-fingers fingers-together"), the second and third toes have extensive skin folds that tightly joins the two toes into a single functional unit. Similarly, Gorillas often have digits 2–5 in both their hands and feet joined by skin folds. [21]
Hominidae/Orangutan
[edit]
In Pongo pygmeus, the surfaces of the CMC are "typically and unequivocally" saddle-shaped, but in other cases the trapezium's ventral overhang might be reduced or swollen; the latter case resembling the condition in gibbons (trapezium surface convex, ball-like). Posterior and anterior oblique ligaments are present, but no "specialized lateral ligament". Capsule is "overlaid laterally by the bony prepollex receiving part of the insertion of the tendon of abductor longus pollicis." [20]
Homininae/Gorilla
[edit]
| “ | Despite decades of debate, it remains unclear whether human bipedalism evolved from a terrestrial knuckle-walking ancestor or from a more generalized, arboreal ape ancestor.
[...] variation among African ape wrist morphology can be clearly explained if we accept the likely independent evolution of 2 fundamentally different biomechanical modes of knuckle-walking:
The presence of purported knuckle-walking features in the hominin wrist can thus be viewed as evidence of arboreality, not terrestriality, and provide evidence that human bipedalism evolved from a more arboreal ancestor occupying the ecological niche common to all living apes. |
” |
| — Kivella & Schmitt 2009 , Independent evolution of knuckle-walking in African apes shows that humans did not evolve from a knuckle-walking ancestor, PNAS[22] | ||
The joint surfaces of the gorilla CMC are saddle-shaped like in human hands and the surface on the trapezium is wider than in chimps, approaching the condition in human hands. The gorilla trapezium has a "striking and unique specialization": adjacent to/behind the dorsal tubercle is a "massive bony apophysis [...] mimicking it"; it extends proximally to form a "supernumary articulation with the tubercle of the scaphoid." "It seems likely that the bony process is derived from the prepollex, and, on occasion, may persist as a separate bony element." [20]
Hominini/Chimps
[edit]
Pan and homo lineages diverged about 5-7 million years ago, and chimpanzees, genetically our nearest living relative, closely resemble the most ancient hominid fossils from that time.
Chimpanzee hands have elongated fingers, metacarpals and carpals with small, weak, and relatively immobile primate thumbs. The third and fourth metacarpals absorb the largest compressive forces during knuckle-walking, and are the most robust bones in the chimpanzee hand. To withstand stresses during arboreal locomotion, the proximal and intermediate phalanges are curved towards the palm. In contrast to the broad human apical tufts, the tips of the chimpanzee fingers are cone-shaped. In the palm, a transversal skin crease reflects the equally arranged metacarpophalangeal joints. The bones of the thumb are slender and short and the thumb muscles intrinsic to the hand are small.
Chimpanzees use a hook grip for suspension from horizontal supports, and a diagonal hook grip with vertical supports. When the thumb is in contact with the support it fails to squeeze the surface against the palm, and while this grip is used for flailing with sticks, the hand tend to lose its grip when the arm swings forward, mostly due to the weak thumb and its inability to overlap the index finger. [2]
The articular surfaces in the chimp CMC are saddle-shaped like in humans, but the surface on the trapezium is less broad. The palmar surface of the trapezium faces almost directly medially. The posterior and anterior oblique ligaments are also similar but the latter is "of flimsier texture". There are no lateral [carpometacarpal] ligament; where on might have expected it a thin fibrous capsule is covered by the insertions of the abductor pollicis longus tendon. [20]
Pan-Homo LCA
[edit]
| Hominid evolution |
| Cladogram of hominid evolution based on post-molecular phylogeny[23] |
There is no fossil representative of the Pan-Homo last common ancestor, but the morphology of this LCA can be inferred from parsimony and hypothesis about phylogenetic relationships between living and extinct primates. Before molecular data was introduced, it was assumed that great apes and humans formed separate clades (i.e. human linage thus pre-dating the LCA of great apes), a concept based on the interpretation of morphological similarities as shared derived characters (synapomorphy) rather than shared primitive characters (symplesiomorphy). At first glance, the hands of the great apes can indeed appear more similar to each other than to those of modern humans, and, for example, in 1970 Napier proposed that the hands of gorillas and chimpanzees are far to specialized to be ancestral to human hands, and that they thus had "no bearing on the evolution of the human hand". However, substantial molecular and morphological evidence suggests that Pan and Homo are more closely related to one another than either is to Gorilla and that these three are more closely related to one another than either is to Pongo. Furthermore, the same evidence indicates that the Pan-Homo LCA existed 8-4 mya, the African apes-Homo LCA 10-6 mya, and a LCA including Orangutan some 18 mya. [24]
A parsimonious interpretation of the present hominid phylogeny indicates that the hand morphology of all extant great apes are homologous. Hand features shared by Pongo and African apes, but not non-hominids primates, were present in the hands of the Hominidae-Homininae LCA. Shared upper limb features related to suspensory behaviours can be homoplastic, and if so, inferences regarding some of the hand features of the Hominidae LCA would require adjustment. Parsimony and many phenetic similarities between modern human hands and those of African apes (rather than Asian apes) suggests it is more likely the hand of the Pan-Homo LCA resembled that of an African ape. [24]
Pre-bipedal locomotor mode -- whether the Pan-Homo LCA was a terrestrially adapted knuckle-walker or a primarily arboreal climber/clamberer...
[...]
- 17 osteological features most likely present in the hands of the Pan-Homo LCA[25]
| Thumb and fingers | |||||
|---|---|---|---|---|---|
| Feature | Non-hominid primates |
Pongo | Gorilla | Pan | Homo |
| Finger length relative thumb | Long | Short | |||
| Shape of proximal phalanges | Curved dorso- palmary |
Straight | |||
| Shaft of proximal phalanges Flexor sheaths |
Robust Marked |
Gracile Weak | |||
| Apical tufts of distal phalanges | Narrow | Broad | |||
| First metacarpal | Gracile | Robust | |||
| Wrist and carpometacarpal joints | |||||
| Feature | Non-hominid primates |
Pongo | Gorilla | Pan | Homo |
| Scaphoid and os centrale | Separate bones |
Fused | |||
| Surfaces of CMC in opposable thumb | Strongly curved | Weakly curved | |||
| Art. surf. of trapezium on scaphoid extends onto scaphoid tubercle |
No | Yes | |||
| Orientation of CMC 2/trapezium joint | radio-ulnarly | proximo-distally | |||
| Trapezoid | Wedge-shaped | Boot-shaped | |||
| Art. surf. of scaphoid on trapezoid | Triangular, large | Rectangular, small | |||
| Art. capitate-trapezoid | Dorsal, smaller | Palmar, larger | |||
| Capitate neck | "Waisted" (on radial side) |
Expanded (on radial side) | |||
| CMC 2/capitate joint orientation | radio-ulnar | proximo-distal | |||
| Styloid proc. at 3rd MC base | Absent | Large | |||
| Midcarpal joint (capitate-hamate) | Narrower | Broader | |||
| Pisiform shape | Longer, rod-shaped | Shorter, pea-shaped | |||
| “ |  Proconsul: Compared to estimated body size, the manual phalanges of Ar. ramidus and Gorilla are long relative to those of the Miocene ape Proconsul. They are relatively even more elongate in Pan, but dramatically abbreviated in Homo. These conclusions are supported by similar calculations using the means of observed body mass. There is no evidence that the manual phalanges of Au. afarensis were elongated relative to those of Ar. ramidus. [26] Ardipithecus ramidus: Its hand was unpredictably unique: Not only was its thumb musculature robust, unlike that of an ape, but its midcarpal joint (in the wrist) allowed the wrist to bend backward to a great degree, enhancing its ability to move along tree branches on its palms. None of the changes that apes have evolved to stiffen their hands for suspension and vertical climbing were present, so its locomotion did not resemble that of any living ape. [27] Viewed in the context of relative limb length patterns, as well as the anatomical details of the hand, the short Mcs of Ar. ramidus strongly suggest that Pan and Gorilla independently acquired elongate Mcs as a part of an adaptation to vertical climbing and suspensory locomotion. Elongation of Mc2-5 in African apes demanded heightened resistance to torsion and consequent fixation of the carpometacarpal joints within the central joint complex (CJC). [26] The thumb metacarpal of [Ardi] was more aptly proportioned for manual grasping than are those of extant apes. [...] Ar. ramidus greatly illuminates the natural history of the thumb in higher primates. Its robusticity in hominids, while certainly enhanced during the past 3 million years, is nevertheless at least partially primitive. In contrast, in taxa adapted to vertical climbing and suspension, lengthening of the palm has become so dominant as to eclipse some of the thumb’s function, a condition that has reached its apogee in Ateles and, to a lesser extent, large-bodied extant apes. These findings strongly suggest that the target of recently discovered major cis-regulatory modification of gene expression in the first ray was not manual but pedal—it is the human hallux, not our largely primitive pollex, that is highly derived. |
” |
| — Lovejoy et al. 2009, Abstract, The Great Divides | ||
-
Ardipithecus finger bones
-
Ardiphitecus —complete skeleton
-
Proconsul skeleton
-
Reconstructed Proconsul skeleton
-
Spider Monkey hand
-
Ateles hybridus
 Media related to Ardipithecus at Wikimedia Commons
Media related to Ardipithecus at Wikimedia Commons
Homo
[edit]- Human hand length proportions are largely plesiomorphic, in the sense that they more closely resemble the relatively short-handed Miocene apes than the elongated hand pattern of extant hominoids. The human complex repertoire of manual grips is possible thanks to human intrinsic manual proportions, i.e. a long thumb relative to the rest of the hand. On the contrary, extant apes possess relatively long hands with a short thumb, in which the musculature is poorly developed.
| “ | It seems improbable that the tremendous dexterity of the human hand can be explained solely by differences in anatomical factors given that the structural anatomy of the hands of different primates seems similar. This is not to say, however, that anatomical differences do not contribute. For example, the human thumb is much longer, relative to the index finger, than the chimpanzee thumb. This allows humans to grasp small objects precisely between the distal pads of the thumb. Similarly, the greater independence of finger movements in humans compared to monkeys arises, in part, from differences in the passive biomechanical connections among tendons. Humans have more individuated muscles and tendons with which to control the digits.
In addition to structural factors, a major contributor to differences in hand movement capacity among primates, and between primates and lower mammals, is the neural machinery underlying hand movement. Compared to lower mammals, primates have evolved extensive cerebral cortical systems for controlling the hand and the corticospinal pathways have taken on an increasingly dominant role in controlling movement. Moreover, in primates the corticospinal tracts include direct connections between neurons in cortical motor areas and spinal motorneurons. Through these corticomotoneuronal connections, the cerebral cortex possesses monosynaptic control over motorneurons whose axons connect, in particular, with the hand muscles. In effect, these direct connections have moved the hand ‘‘closer’’ to the cerebral cortex. Furthermore, through cortical motor areas the corticospinal tracts provide rapid access to the hand from most other cortical areas and from subcortical structures, including the cerebellum and the basal ganglia, tightly involved in motor control. |
” |
| — Flanagan & Johansson 2002, hand Movements, Encyclopedia of the human brain[29] | ||
| “ | Given that all primates possess the ability to dexterously reach, grasp and manipulate objects with their hands, why is it that only humans have developed such an extensive and universal material culture?
[...] ...the ways in which we skilfully interact with tools and other artefacts are highly context dependent, and aspects of this context are not specified by the physical properties of the task or actor's body. Though certainly necessary, sensory–motor processes alone are therefore insufficient to account for this flexibility. These skills are also influenced by conceptual knowledge about objects and their functions, the actor's intended goals and interpretations of prevailing task demands. These various sources of sensory–motor and cognitive information are somehow integrated to form internal representations that constrain and guide manual praxis, engendering a flexibility that is absent even in the tool-using behaviours of our nearest living relatives. |
” |
| — Frey 2008 , Tool use, communicative gesture and cerebral asymmetries in the modern human brain[30] | ||
| “ | Understanding gestures cannot only consist of describing chains of interconnected reflexes and the particular actions they make possible but must conceptualize the kind of functions they were driven to implement by natural selection. In other words, each step in their emergence must be conceived as a kind of photographic negative of the environmental constraints which selected across time the features which are combined in their overall adaptiveness. This view does not imply that the whole system obeys a preconceived plan, a blueprint which would have, from the beginning, taken into consideration a range of functions which it was designed to serve. Indeed, when we deal with gestures and the anatomical apparatus that sustains them, identifying a “beginning”, a starting point, would be a daunting task, if only because there cannot be a single point of origin for any organ but distributed factors from which viable patterns emerged through natural selection in the context of whole organisms and populations.
A variety of evolutionary pressures can converge toward morphologies with a variety of functions. Efficiency for survival in a particular environment is the key, rather than the overall consistency of the design. Old morphologies can be coopted by new emergent functions when the context is modified, a phenomenon which some evolutionists call “exaptation”. Such a theoretical frame must be considered for understanding the human arm and hand. Appendages, limbs and digits are not confined to primates, not even to mammals. The evolutionary snapshot of the last two million years can be understood only if assessed with respect to a much deeper “history”. |
” |
| — Bouissac 2004, Gestures in Evolutionary Perspective[31] | ||
| “ | Though other species of primates also use tools, humans appear unique in their capacity to understand the causal relationship between tools and the result of their use. In a comparative fMRI study, we scanned a large cohort of human volunteers and untrained monkeys, as well as two monkeys trained to use tools, while they observed hand actions and actions performed using simple tools. In both species, the observation of an action, regardless of how performed, activated occipitotemporal, intraparietal, and ventral premotor cortex, bilaterally. In humans, the observation of actions done with simple tools yielded an additional, specific activation of a rostral sector of the left inferior parietal lobule (IPL). This latter site was considered human-specific, as it was not observed in monkey IPL for any of the tool videos presented, even after monkeys had become proficient in using a rake or pliers through extensive training. In conclusion, while the observation of a grasping hand activated similar regions in humans and monkeys, an additional specific sector of IPL devoted to tool use has evolved in Homo sapiens, although tool-specific neurons might reside in the monkey grasping regions. These results shed new light on the changes of the hominid brain during evolution. | ” |
| — Peeters et al. 2009 , The Representation of Tool Use in Humans and Monkeys: Common and Uniquely Human Features[32] | ||
- Further reading
- McHenry, Henry M. (1983). "The capitate of Australopithecus afarensis and A. africanus" (PDF). American Journal of Physical Anthropology. 62 (2): 187–98. doi:10.1002/ajpa.1330620208. PMID 6418011.
In overall shape the bones are more like H. sapiens than other extant hominids, although they are uniquely different. The two A. afarensis capitates provide no evidence that there are two postcranial morphotypes at Hadar. Available evidence shows that A. afarensis and A. africanus are strikingly similar postcranially. The morphological differences between the capitate of Australopithecus and H. sapiens may relate to the retention of climbing ability and an absence of certain grip capabilities in these early hominids.
- Susman, RL (May 6, 1988). "Hand of Paranthropus robustus from Member 1, Swartkrans: fossil evidence for tool behavior". Science. 240 (4853): 781–4. doi:10.1126/science.3129783. PMID 3129783.
New hand fossils from Swartkrans (dated at about 1.8 million years ago) indicate that the hand of Paranthropus robustus was adapted for precision grasping. Functional morphology suggests that Paranthropus could have used tools, possibly for plant procurement and processing. The new fossils further suggest that absence of tool behavior was not responsible for the demise of the "robust" lineage. Conversely, these new fossils indicate that the acquisition of tool behavior does not account for the emergence and success of early Homo.
Additional images
[edit]-
Epidermal ridges in a Ring-tailed lemur
-
Front paws of a female Eastern Grey Kangaroo
See also
[edit]- Prepollex: Cochranella oyampiensis, Nuptial pad, Amplexus
- Synapomorphy, Homology, Homoplasty
- Homoiology Homoiological features mimic potential homologies or homoplasies through phenotypic plasticity via common epigenetic factors such as a common developmental program coupled with a similar mechanical environment. (Tacheri 2008, p 547).
- Carpus and tarsus of land vertebrates
- Equine forelimb anatomy
- In humans, the apical phalangeal tufts are flat and wide mediolaterally. This enlargement enhanced friction between the volar pad of the tip of the fingers and early tools such as lithic cones and hammer-stones. In other primates, especially suspensory arboreal species, the apical tufts were smaller.
- Mittra ES, Smith HF, Lemelin P, Jungers WL. (2007 Dec). "Comparative morphometrics of the primate apical tuft". Am J Phys Anthropol. 4 (134): 449–59. doi:10.1002/ajpa.20687. PMID 17657781.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link)
- Mittra ES, Smith HF, Lemelin P, Jungers WL. (2007 Dec). "Comparative morphometrics of the primate apical tuft". Am J Phys Anthropol. 4 (134): 449–59. doi:10.1002/ajpa.20687. PMID 17657781.
- Timelines
- Timeline of human evolution
- Timeline of evolution
- Template for simplified timelines: {{include timeline}}
- Advanced example: {{Timeline geological timescale}}
Notes
[edit]- ^ Bouissac 2004, p. 4
- ^ a b Young 2003
- ^ Marzke, Massey University
- ^ "Human Evolution: Tools, hands, and heads in the Pliocene and Pleistocene". Encyclopædia Britannica. Retrieved December 2009.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Tocheri et al. 2008
- ^ Brüser, Gilbert & Brunelli 1999, p. 167
- ^ Thies, Monte L. "The Appendicular Skeleton". Sam Houston State University. Retrieved May 2010.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Hyman, Libbie Henrietta; Wake, Marvalee H. (1992). Hyman's Comparative Vertebrate Anatomy. University of Chicago Press. ISBN 9780226870137.
- ^ Gough-Palmer, Maclachlan & Routh 2008, p. 510
- ^ Gough-Palmer, Maclachlan & Routh 2008, p. 582
- ^ Gough-Palmer, Maclachlan & Routh 2008, p. 583
- ^ Gough-Palmer, Maclachlan & Routh 2008, p. 504
- ^ Gough-Palmer, Maclachlan & Routh 2008, p. 505
- ^ Gough-Palmer, Maclachlan & Routh 2008, p. 507-8
- ^ Gough-Palmer, Maclachlan & Routh 2008, p. 508-9
- ^ Burton & Burton 2002, pp. 2034–5
- ^ Ankel-Simons 2000, p. 340
- ^ Tocheri et al. 2008, p. 546
- ^ Susman 1979
- ^ a b c d Lewis 1977, pp. 160–61
- ^ Ankel-Simons 2007, p. 342
- ^ Kivella & Schmitt 2009
- ^ Tocheri et al. 2008, p. 546
- ^ a b Tocheri et al. 2008, pp. 545–6
- ^ Tocheri et al. 2008, pp. 546, 548
- ^ a b Lovejoy et al. 2009, pp. 101–102
- ^ Lovejoy et al. 2009, Abstract
- ^ Almécija, Moyà-Solà & Alba 2010
- ^ Flanagan & Johansson 2002, hand Movements
- ^ Frey 2008
- ^ Bouissac 2004
- ^ Peeters et al. 2009
References
[edit]- Almécija, Sergio (2009). "Evolution of the hand in Miocene apes: implications for the appearance of the human hand (PhD Thesis)" (PDF). Universitat Autònoma de Barcelona.
{{cite journal}}: Cite journal requires|journal=(help) - Almécija, S; Moyà-Solà, S; Alba, DM (2010). "Early Origin for Human-Like Precision Grasping: A Comparative Study of Pollical Distal Phalanges in Fossil Hominins". PLOS ONE. 5 (7): e11727. doi:10.1371/journal.pone.0011727. PMC 2908684. PMID 20661444.
- Ankel-Simons, Friderun (2000). "Hands and Feet". Primate anatomy: an introduction. Academic Press. pp. 300ff. ISBN 0120586703.
- Bouissac, Paul (2004). "Gestures in Evolutionary Perspective" (PDF). Open Semiotics Resource Center. Retrieved March 2010.
{{cite web}}: Check date values in:|accessdate=(help) - Brüser, Peter; Gilbert, Alain; Brunelli, Giovanni R. (1999). "Stability in the first carpometacarpal joint". Finger bone and joint injuries. Taylor & Francis. ISBN 1853176907.
- Burton, Maurice; Burton, Robert (2002). International Wildlife Encyclopedia (3rd ed.). Marshall Cavendish. pp. 2034–2035. ISBN 0761472665.
- Flanagan, J Randall; Johansson, Roland S (2002). "Hand Movements". Encyclopedia of the human brain (PDF). Elsevier Science.
- Frey, Scott H (2008). "Tool use, communicative gesture and cerebral asymmetries in the modern human brain". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1499). Phil. Trans. R. Soc. B: 1951–1957. doi:10.1098/rstb.2008.0008. PMC 2606701. PMID 18292060.
{{cite journal}}: Unknown parameter|month=ignored (help) - Hartwig, Walter Carl (2002). The primate fossil record. Cambridge University Press. ISBN 0521663156.
- Gough-Palmer, Antony L.; Maclachlan, Jody; Routh, Andrew (March 2008). "Paws for Thought: Comparative Radiologic Anatomy of the Mammalian Forelimb". Radiographics. 28 (2): 501–10. doi:10.1148/rg.282075061. PMID 18349453.
{{cite journal}}: CS1 maint: date and year (link) (PDF) - Kivella, Tracy L.; Schmitt, Daniel (2009). "Independent evolution of knuckle-walking in African apes shows that humans did not evolve from a knuckle-walking ancestor". PNAS. 106 (34): 14241–14246. doi:10.1073/pnas.0901280106. PMC 2732797. PMID 19667206.
{{cite journal}}: Unknown parameter|month=ignored (help) - Lewis, O J (April 1964). "The homologies of the mammalian tarsal bones". J Anat. 98 (Pt 2): 195–208. PMC 1261275. PMID 14154422.
{{cite journal}}: CS1 maint: date and year (link) - Lewis, O J (February 1977). "Joint remodelling and the evolution of the human hand". J Anat. 123 (Pt 1): 157–201. PMC 1234261. PMID 402345.
{{cite journal}}: CS1 maint: date and year (link) - Lovejoy, C. Owen; Suwa, Gen; Simpson, Scott W.; Matternes, Jay H. (October 2009). "The Great Divides: Ardipithecus ramidus Reveals the Postcrania of Our Last Common Ancestors with African Apes". Science. 326 (5949): 73, 100–106. doi:10.1126/science.1175833. PMID 19810199. S2CID 19629241.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - Marzke, MW; Marzke, RF (July 2000). "Evolution of the human hand: approaches to acquiring, analysing and interpreting the anatomical evidence". J Anat. 197 (Pt 1): 121–40. doi:10.1046/j.1469-7580.2000.19710121.x. PMC 1468111. PMID 10999274.
{{cite journal}}: CS1 maint: date and year (link) - Marzke, Mary. "Evolution of the hand and bipedality". Massey University, NZ. Retrieved December 2009.
{{cite web}}: Check date values in:|accessdate=(help) - Marzke, Mary (1999). "Evolution of the hand and bipedality". In Lock, Andrew; Peters, Charles R. (eds.). Handbook of human symbolic evolution. Wiley-Blackwell. ISBN 0631216901.
- Peeters, R.; Simone, L.; Nelissen, K.; Fabbri-Destro, M. (2009). "The Representation of Tool Use in Humans and Monkeys: Common and Uniquely Human Features". The Journal of Neuroscience. 29 (37): 11523–11539. doi:10.1523/JNEUROSCI.2040-09.2009. PMC 6665774. PMID 19759300.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - Putz, RV; Tuppek, A. (November 1999). "Evolution of the hand". Handchir Mikrochir Plast Chir. 31 (6): 357–61. doi:10.1055/s-1999-13552. PMID 10637723. Retrieved December 2009.
{{cite journal}}: Check date values in:|accessdate=(help)CS1 maint: date and year (link) - Susman, Randall L. (1979). "Comparative and functional morphology of hominoid fingers". American Journal of Physical Anthropology. 50 (2): 215–36. doi:10.1002/ajpa.1330500211. PMID 443358.
- Schwartz, Jeffrey; Yamada, Tadasu K. (1998). "Carpal Anatomy and Primate Relationships" (PDF). Anthropological Science. 106 (Supplement): 47–65. doi:10.1537/ase.106.Supplement_47.
- Tocheri, Matthew W.; Orr, Caley M.; Jacofsky, Marc C.; Marzke, Mary W. (2008). "The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo". J. Anat. 212 (4): 544–562. doi:10.1111/J.1469-7580.2008.00865.X. PMC 2409097. PMID 18380869. (Abstract, PubMed) (PDF, Smithsonian)
- Young, Richard W (January 2003). "Evolution of the human hand: the role of throwing and clubbing". Journal of Anatomy. 202 (1): 165–74. doi:10.1046/j.1469-7580.2003.00144.x. PMC 1571064. PMID 12587931.
{{cite journal}}: CS1 maint: date and year (link) PDF





























































