Pharmacokinetics of estradiol
 | |
| Clinical data | |
|---|---|
| Routes of administration | • By mouth (tablet) • Sublingual (tablet) • Intranasal (nasal spray) • Transdermal (patch, gel, cream, emulsion, spray) • Vaginal (tablet, cream, suppository, insert, ring) • IM injection (oil solution) • SC injection (aq. soln.) • Subcutaneous implant |
| Drug class | Estrogen; Antigonadotropin |
| Pharmacokinetic data | |
| Bioavailability | Oral: 5% (0.1–12%)[1][2] SL: 10% (in marmosets)[3] IM: 100%[4] |
| Protein binding | ~98%:[1][5] • Albumin: 60% • SHBG: 38% • Free: 2% |
| Metabolism | Liver (via hydroxylation, sulfation, glucuronidation) |
| Metabolites | Major (90%):[1] • Estrone • Estrone sulfate • Estrone glucuronide • Estradiol glucuronide |
| Elimination half-life | Oral: 13–20 hours[1] Sublingual: 8–18 hours[6] Transdermal (gel): 37 hours[7] IM (as EV): 4–5 days[4] IM (as EC): 8–10 days[8] IV (as E2): 0.5–2 hours[4][9] |
| Excretion | Urine: 54%[1] Feces: 6%[1] |
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.[10][11][12]
Estradiol is a naturally occurring and bioidentical estrogen, or an agonist of the estrogen receptor, the biological target of estrogens like endogenous estradiol.[10] Due to its estrogenic activity, estradiol has antigonadotropic effects and can inhibit fertility and suppress sex hormone production in both women and men.[13][14] Estradiol differs from non-bioidentical estrogens like conjugated estrogens and ethinylestradiol in various ways, with implications for tolerability and safety.[10]
Estradiol can be taken by mouth, held under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.[10]
Routes of administration
[edit]Estradiol can be taken by a variety of different routes of administration.[10] These include oral, buccal, sublingual, intranasal, transdermal (gels, creams, patches), vaginal (tablets, creams, rings, suppositories), rectal, by intramuscular or subcutaneous injection (in oil or aqueous), and as a subcutaneous implant.[10] The pharmacokinetics of estradiol, including its bioavailability, metabolism, biological half-life, and other parameters, differ by route of administration.[10] Likewise, the potency of estradiol, and its local effects in certain tissues, most importantly the liver, differ by route of administration as well.[10] In particular, the oral route is subject to a high first-pass effect, which results in high levels of estradiol and consequent estrogenic effects in the liver and low potency due to first-pass hepatic and intestinal metabolism into metabolites like estrone and estrogen conjugates.[10] Conversely, this is not the case for parenteral (non-oral) routes, which bypass the intestines and liver.[10]
Different estradiol routes and dosages can achieve widely varying circulating estradiol levels (see the table below).[10] For purposes of comparison with normal physiological circumstances, menstrual cycle circulating levels of estradiol in premenopausal women are 40 pg/mL in the early follicular phase, 250 pg/mL at the middle of the cycle, and 100 pg/mL during the mid-luteal phase.[15] Mean integrated levels of circulating estradiol in premenopausal women across the whole menstrual cycle are in the range of 80 to 150 pg/mL, according to some sources.[16][17][18] In postmenopausal women, circulating levels of estradiol are below 15 pg/mL.[10][15] During normal human pregnancy, estrogen production increases progressively and extremely high estrogen levels are attained.[19] Estradiol levels range from 1,000 to 40,000 pg/mL across pregnancy,[20] are on average 25,000 pg/mL at term, and reach levels as high as 75,000 pg/mL in some women.[21]
| Route | Ingredient | Form | Dose[b] | Brand names[c] |
|---|---|---|---|---|
| Oral | Estradiol | Tablet | 0.1, 0.2, 0.5, 1, 2, 4 mg | Estrace, Ovocyclin |
| Estradiol valerate | Tablet | 0.5, 1, 2, 4 mg | Progynova | |
| Transdermal | Estradiol | Patch | 14, 25, 37.5, 50, 60, 75, 100 µg/d | Climara, Vivelle |
| Gel pump | 0.06% (0.52, 0.75 mg/pump) | Elestrin, EstroGel | ||
| Gel packet | 0.1% (0.25, 0.5, 1.0 mg/pk.) | DiviGel, Sandrena | ||
| Emulsion | 0.25% (25 µg/pouch) | Estrasorb | ||
| Spray | 1.53 mg/spray | Evamist, Lenzetto | ||
| Vaginal | Estradiol | Tablet | 10, 25 µg | Vagifem |
| Cream | 0.01% (0.1 mg/gram) | Estrace | ||
| Insert | 4, 10 µg | Imvexxy | ||
| Ring | 2 mg/ring (7.5 µg/d, 3 mon.) | Estring | ||
| Estradiol acetate | Ring | 50, 100 µg/d, 3 months | Femring | |
| Injection[d] | Estradiol | Microspheres | 1 mg/mL | Juvenum E |
| Estradiol benzoate | Oil solution | 0.167, 0.2, 0.333, 1, 1.67, 2, 5, 10, 20, 25 mg/mL | Progynon-B | |
| Estradiol cypionate | Oil solution | 1, 3, 5 mg/mL | Depo-Estradiol | |
| Estradiol valerate | Oil solution | 5, 10, 20, 40 mg/mL | Progynon Depot | |
| Implant | Estradiol | Pellet | 20, 25, 50, 100 mg, 6 mon. | Estradiol Implants |
Notes and sources:
| ||||
| Route | Dose (mg) |
Time measured |
ΔE2 levels (pg/mL) |
ΔE1 levels (pg/mL) |
E2:E1 ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| Oral |
1 2 4 |
12 h 3 h 6 h |
+25 +40 +50 |
+150 +250 +500 |
0.15 0.16 0.10 | ||||
| Sublingual |
1 0.5 0.5 0.5 |
1 h 1 h 1 h 1 h |
+450 +250 +750 +75 |
+160 +85 +250 +24 |
3 3 3 3 | ||||
| Intranasal | 1 | 1 h | +110 | +110 | 1.0 | ||||
| Topical (gel) |
3 3/d 3/2 d |
5 h 12–20 h 12 h 36 h |
+70 +45–279 +300–1310 +20–179 |
+50 +31–230 +24–110 +120–500 |
0.4 1 1 1 | ||||
| Vaginal (cream) | 0.5 1.0 |
3 h 3 h |
+830 +800 |
+150 +150 |
5.0 5.0 | ||||
| Rectal | 1 | 3 h | +620 | +120 | 5.0 | ||||
| Intramuscular inj. (oil soln.) |
EB: 5 EV: 5 EC: 5 EU: 100 PEP: 320 |
1.8, 2.4 da 2.2, 2.7 da 3.9, 5.1 da 1 d 16, 25 da |
940b 667b 338b 500b 270b |
343b 324b 145b ND 1000b |
2.7 2.1 2.3 ND 0.27 | ||||
| Intravenous inj. | 0.3 | 5 min | 8321b | 960b | 8.7 | ||||
| Footnotes: a = Tmax for E2, E1 levels. b = Actual levels (not change). Sources: See template. | |||||||||
Oral administration
[edit]Absorption and bioavailability
[edit]The oral bioavailability of estradiol is very low.[2][36][37] This is due to the fact that estradiol is poorly soluble in water, which limits its dissolution and absorption, and is additionally subject to extensive metabolism during the first pass through the intestines and liver.[3][38] Estradiol is micronized and/or conjugated with an ester, as in estradiol valerate or estradiol acetate, to improve its oral bioavailability and potency.[2][36][37] Micronization decreases the particle size of estradiol crystals and hence increases the surface area for absorption, thereby improving the rate and extent of absorption.[39][3][40] In addition, there is an improvement in metabolic stability.[3][40] Oral micronized estradiol consists of more than 80% of estradiol particles micronized to a size smaller than 20 μm in diameter, or to about 1 to 3 μm on average.[41][42][40][43] All oral formulations of estradiol available today are micronized,[36] and oral estradiol valerate tablets also seem to be micronized.[44]
Oral non-micronized estradiol and oral micronized estradiol do not appear to have ever been directly compared in a study.[45][42][46][47] Both have been assessed independently however, and have been found to produce significant estrogenic effects.[45][42][46][48] Micronization of other poorly water-soluble steroids such as spironolactone and norethisterone acetate has been found to increase their potency by several-fold.[49][50][51][52][53] In accordance, studies of the amount of oral estradiol necessary for endometrial proliferation in women have reported a total dose of 60 mg for micronized estradiol[54] relative to 120 to 300 mg or more for non-micronized estradiol.[55][56][57] As such, micronization has been said to substantially improve the potency of oral estradiol.[40]

A study compared different particle sizes of oral micronized estradiol.[38][58][59] A preparation with the smallest particles (mainly <0.6 μm) was found to have the most rapid absorption and the highest bioavailability.[38][59] However, a sharp peak in estradiol levels, without an accompanying rise in estrone levels, was observed during the first 2 hours with this particle size.[38][59] It was suggested that the smallest estradiol particles may have been absorbed by the lymphatic system, partially bypassing first-pass metabolism and resulting in very high initial estradiol levels.[38] The preparations with the larger particle sizes (mainly <3.5 μm and <20 μm) were found to be absorbed more slowly, without a pronounced initial peak in estradiol levels.[38][59] Levels of estradiol were more even and similar to physiological levels with these particle sizes.[38][59] Differences in area-under-the-curve estradiol levels with the different particle sizes were relatively small.[38] As such, micronization may improve absorption but does not necessarily improve therapeutic effect.[59]
Micronized estradiol is rapidly and completely absorbed with oral administration.[3][12] This is true for oral doses of 2 mg and 4 mg, but absorption was found to be incomplete for an oral dose of 8 mg.[3][60] This dose showed 76% of the expected bioavailability based on dose proportionality and area-under-the-curve levels, indicating a small deviation from linearity.[3][60] The absolute bioavailability of oral micronized estradiol is approximately 5%, with a possible range of 0.1% to 12%.[1][2][59] As such, the bioavailability of oral estradiol is very low even with micronization.[59] There is high interindividual variability in the levels of estradiol achieved with oral estradiol, which is likely related to the high first-pass effect.[12] This variation has been reported to be in the range of 28 to 127%, or about 4.6-fold maximal difference in levels between individuals, in terms of mean area-under-the-curve levels of estradiol.[12]
In postmenopausal women, a dosage of 1 mg/day oral micronized estradiol has been found to produce circulating concentrations of 30 to 50 pg/mL estradiol and 150 to 300 pg/mL estrone, while a dosage of 2 mg/day has been found to result in circulating levels of 50 to 180 pg/mL estradiol and 300 to 850 pg/mL estrone.[15] A study of oral micronized estradiol in transgender women found that mean estradiol levels across a dosage range of 1 to 8 mg/day were about 50 pg/mL at 1 mg/day, 100 pg/mL at 4 mg/day, and 150 pg/mL at 8 mg/day, with a wide degree of variation.[61] In another study, mean estradiol levels at steady state with 4 mg/day and 6 mg/day oral micronized estradiol were approximately 180 pg/mL and 265 pg/mL, respectively.[62] A study that used high to very high-dose oral micronized estradiol in postmenopausal women found that steady-state estradiol levels with 6 mg/day were about 300 pg/mL and with 30 mg/day were about 2,400 pg/mL.[63]
Estradiol valerate is rapidly hydrolyzed into estradiol in the intestines.[10][64][65] For this reason, oral estradiol and oral estradiol valerate have very similar pharmacokinetics.[10][64][65] Due to the presence of its valeric acid ester and differences in molecular weight, estradiol valerate contains about 76% of the same amount of estradiol by weight.[66][67][68][69] As a result, 2 mg oral estradiol valerate produces equivalent estradiol levels to about 1.5 mg oral estradiol.[66][67][68][69]
| Compound | Dosage for specific uses (mg usually)[a] | ||||||
|---|---|---|---|---|---|---|---|
| ETD[b] | EPD[b] | MSD[b] | MSD[c] | OID[c] | TSD[c] | ||
| Estradiol (non-micronized) | 30 | ≥120–300 | 120 | 6 | - | - | |
| Estradiol (micronized) | 6–12 | 60–80 | 14–42 | 1–2 | >5 | >8 | |
| Estradiol valerate | 6–12 | 60–80 | 14–42 | 1–2 | - | >8 | |
| Estradiol benzoate | - | 60–140 | - | - | - | - | |
| Estriol | ≥20 | 120–150[d] | 28–126 | 1–6 | >5 | - | |
| Estriol succinate | - | 140–150[d] | 28–126 | 2–6 | - | - | |
| Estrone sulfate | 12 | 60 | 42 | 2 | - | - | |
| Conjugated estrogens | 5–12 | 60–80 | 8.4–25 | 0.625–1.25 | >3.75 | 7.5 | |
| Ethinylestradiol | 200 μg | 1–2 | 280 μg | 20–40 μg | 100 μg | 100 μg | |
| Mestranol | 300 μg | 1.5–3.0 | 300–600 μg | 25–30 μg | >80 μg | - | |
| Quinestrol | 300 μg | 2–4 | 500 μg | 25–50 μg | - | - | |
| Methylestradiol | - | 2 | - | - | - | - | |
| Diethylstilbestrol | 2.5 | 20–30 | 11 | 0.5–2.0 | >5 | 3 | |
| DES dipropionate | - | 15–30 | - | - | - | - | |
| Dienestrol | 5 | 30–40 | 42 | 0.5–4.0 | - | - | |
| Dienestrol diacetate | 3–5 | 30–60 | - | - | - | - | |
| Hexestrol | - | 70–110 | - | - | - | - | |
| Chlorotrianisene | - | >100 | - | - | >48 | - | |
| Methallenestril | - | 400 | - | - | - | - | |
| Estrogen | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | ? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
Sources and footnotes
Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (hot flashes/gonadotropins). Sources: See template. | ||||||||||
Metabolism and elimination
[edit]When taken orally, about 95% of a dose of estradiol is metabolized in the intestines and liver into estrone and estrogen conjugates such as estrone sulfate, estrone glucuronide, and estradiol sulfate, among others, prior to entering the circulation.[3][88][89][90] As a result, circulating estrone and estrogen conjugate levels are markedly elevated, in a highly unphysiological manner, with oral estradiol.[88][91] Whereas the ratio of circulating estradiol to estrone is about 1:1 in premenopausal women and with transdermal estradiol, oral estradiol produces a ratio of about 1:5 on average and as high as 1:20 in some women.[1][10][92][60] In addition, whereas levels of estradiol with menopausal replacement dosages of oral estradiol are in the range of the follicular phase of the normal menstrual cycle, levels of estrone resemble those during the first trimester of pregnancy.[93][94] Moreover, whereas normal physiological estrone sulfate levels are 10 to 25 times higher than those of estradiol and estrone in premenopausal women,[95] levels of estrone sulfate with oral estradiol are an additional 8 to 20 times higher than normal premenopausal or postmenopausal estrone sulfate levels.[91][96][97] One study found that estrone sulfate levels were 200-fold higher than estradiol levels with 2 mg/day oral micronized estradiol or oral estradiol valerate, and estrone sulfate levels can reach up to nearly 1,000-fold higher concentrations than estradiol in some cases.[10][12] In contrast to oral estradiol, due to the lack of the first pass, an excess in estrone and estrogen conjugate levels does not occur with transdermal estradiol or other parenteral estradiol routes.[88][91]
The transformation of estradiol into estrone and estrogen conjugates is reversible.[10] As such, these metabolites can be converted back into estradiol.[10] About 15% of orally administered estradiol is transformed into estrone and 65% into estrone sulfate.[12] About 5% of estrone and 1.4% of estrone sulfate is converted back into estradiol.[12] An additional 21% of estrone sulfate is converted into estrone, whereas transformation of estrone into estrone sulfate is approximately 54%.[12] The interconversion between estradiol and estrone is mediated by 17β-hydroxysteroid dehydrogenases (17β-HSDs),[12] whereas the conversion of estrone into estrone sulfate is mediated by estrogen sulfotransferases (ESTs) and the transformation of estrone sulfate into estrone by steroid sulfatase (STS).[98][99] The metabolic clearance rates and hence blood half-lives of estrogen conjugates like estrone sulfate are much longer than those of estradiol and estrone.[10][12][91] Estrogen conjugates, primarily estrone sulfate, serve as a large circulating reservoir for estradiol, and because of this, they function to greatly extend the biological half-life of oral estradiol.[10][12] As such, the biological half-life of oral estradiol is a composite parameter that is dependent on interconversion between estradiol and estrogen conjugates, as well as on enterohepatic recirculation.[12] Whereas the biological half-life of estradiol given by intravenous injection is about 0.5 to 2 hours, the biological half-life of oral estradiol has a range of 13 to 20 hours due to the large and long-lasting pool of estrogen conjugates that is formed during first-pass metabolism and that serves to continuously replenish circulating estradiol levels.[12][10][9]
First-pass effect and differences from other routes
[edit]The first-pass effect that occurs with oral estradiol results in unusually high levels of estrone and estrogen conjugates in the circulation as well as of estradiol in the liver.[10] These unique properties of oral estradiol result in a number of pharmacological differences relative to the other routes of administration of estradiol.[10]
The high levels of estrone and estrogen conjugates that occur with oral estradiol raise the question of the pharmacodynamic significance of these metabolites.[10] In contrast to estradiol however, estrone has very low activity as an estrogen.[10][100][101] The affinities of estrone for the human ERs and its estrogenic activity have been reported to be approximately 3 to 4% of those of estradiol.[10] In addition, unlike estradiol and estriol, estrone is not accumulated in target tissues.[10] Because estrone can be transformed into estradiol, most of its activity in vivo is actually due to conversion into estradiol.[10] In accordance, doses of oral and transdermal estradiol that achieve similar levels of estradiol have been found, in spite of markedly elevated levels of estrone with oral estradiol but not with transdermal estradiol, to possess equivalent and non-significantly different potency in terms of clinical measures including suppression of LH and FSH levels, inhibition of bone resorption, and relief of menopausal symptoms such as hot flashes.[10][88][102][103][96][104] In addition, estradiol levels were found to correlate with these effects, while estrone levels did not.[88][102] These findings suggest that estrone contributes very little or not at all to the estrogenic potency of estradiol, while also not antagonizing the estrogenic activity of estradiol.[10][88][102][103] This contradicts some cell-free in-vitro research suggesting that high concentrations of estrone might be able to partially antagonize the actions of estradiol.[105][106][107]
On the other hand, it has been suggested by some authors that the high levels of estrone and/or estrone conjugates with oral estradiol may result in excessive estradiol levels in certain tissues such as the breasts and endometrium, due to high expression in these tissues of the requisite enzymes (17β-HSDs and STS) necessary to transform these metabolites back into estradiol.[94][91][111][112] In accordance, circulating levels of estrone sulfate have been found to be positively associated with breast density in postmenopausal women treated with oral estradiol, with 1.3% higher breast density observed for every 1 ng/mL greater level of estrone sulfate.[113][114] Similarly, levels of estradiol, estrone, and estrone sulfate are all strongly associated with the risk of breast cancer in women.[113] Preclinical studies have shown that estrone sulfate, via local transformation into estradiol, stimulates the growth of mammary cancer cells.[115][116]
Due to the first pass through the liver, disproportionate and supraphysiological levels of estrogens occur locally in the liver with oral estradiol.[117][12] These levels are approximately 4- to 5-fold higher than in the circulation, based on differences in hepatic estrogenic potency for oral estradiol relative to transdermal estradiol.[117][10] As a result, there is abnormally high estrogenic signaling in the liver with oral estradiol, and a variety of unphysiological effects on liver protein synthesis result.[10][12] Through modulation of liver protein synthesis, conjugated oral estrogen increases the risk of blood clots,[118] increases circulating levels of a variety of binding proteins including thyroid binding globulin (TBG), cortisol binding globulin (CBG), sex hormone binding globulin (SHBG), growth hormone binding protein (GHBP),[119] insulin-like growth factor-binding proteins (IGFBPs),[120] and copper binding protein (CBP),[89][121] suppresses growth hormone (GH)-mediated insulin-like growth factor 1 (IGF-1) production,[122][123] and produces positive blood lipid changes, among a variety of other effects.[90][124][12] In contrast to oral estradiol, transdermal estradiol has relatively minimal impact on liver protein synthesis.[10] As an example, a study found that 1 mg/day oral estradiol significantly increased SHBG levels by 45%, while 50 μg/day transdermal estradiol increased SHBG levels non-significantly by only 12%.[125][126][127]
In the circulation, approximately 38% of estradiol is reversibly bound to SHBG and 60% is reversibly bound to albumin in women under normal physiological circumstances, with 2 to 3% of total estradiol circulating free or unbound at any given time.[3][2][1] Only estradiol that is free or unbound is able to be enter target cells and hence is biologically active.[1][12]: 249 [17] The increase in SHBG levels with oral estradiol (e.g., +50%) can result in a clinically meaningful increase in the fractions of sex hormones like estradiol and testosterone that are bound to SHBG, whereas this is not the case with typical clinical dosages of transdermal estradiol.[128][17] The increase in the fraction of estradiol bound to SHBG results in a significant decrease in the percentage of free or unbound and hence bioactive estradiol.[1][17] As a result, the bioavailability and potency of oral estradiol may be diminished relative to parenteral estradiol routes by some amount.[17][1] However, a study found that the free fraction of estradiol was similar with doses of oral and topical estradiol that resulted in equivalent total estradiol levels.[129]
Experimental oral formulations
[edit]Estradiol decanoate, estradiol cyclooctyl acetate, estradiol 3-saccharinylmethyl ether, and EC508 (estradiol 17β-(1-(4-(aminosulfonyl)benzoyl)-L-proline)) are estradiol esters and novel oral forms of estradiol that have been developed with improved properties, such as greater bioavailability and reduced first-pass effect.[130][131][132][133][134][135][136][137][138][139][140][141] Estradiol decanoate and estradiol cyclooctyl acetate were studied for potential use in menopausal hormone therapy and birth control pills but were never marketed.[130][131][132][133][134][135][136][137] EC508 is currently under active development for use in menopausal hormone therapy.[140][141]
Graphs
[edit]-
Estradiol levels after a single oral dose of 2, 4, or 8 mg estradiol in premenopausal women.
-
Estradiol and estrone levels following a single 2 mg dose of oral estradiol in postmenopausal women.
-
Mean estradiol levels during 1 to 8 mg/day oral estradiol therapy alone or in combination with 100 to 200 mg/day spironolactone in transgender women.
-
Percent change in estradiol (E2), estrone (E1), LH, and FSH levels over a 24-hour period following a single dose of 2 mg oral estradiol in women.
-
Levels of estradiol, estrone, and estrone sulfate following a single 2 mg oral dose of estradiol valerate in postmenopausal women.
-
Estradiol levels after a single dose of 2 mg oral estradiol or 2 mg oral estradiol valerate and with continuous administration of 2 mg/day oral estradiol or 2 mg/day oral estradiol valerate (at steady state) in postmenopausal women.
Buccal administration
[edit]
Estradiol has been studied for use by buccal administration.[10][72][142][143][144][145][146][147] Preclinical studies of buccal estradiol have also been conducted.[148][149][150][151] Buccal and sublingual administration of estradiol have similar characteristics.[10]
Administration of a troche (lozenge) containing 0.25 mg estradiol via the buccal route resulted in peak estradiol levels of about 450 pg/mL at 1 hour post-dose in postmenopausal women.[10][142] Following this, estradiol levels decreased to about 60 pg/mL at 4 hours post-dose and to about 15 pg/mL at 12 hours post-dose.[10][142] With continuous twice daily administration of 0.25 mg estradiol (0.5 mg/day total) via the buccal route once every 12 hours, peak estradiol levels at steady state after the last dose were about 500 pg/mL.[10][142]
Sublingual administration
[edit]Estradiol tablets can be taken sublingually instead of orally.[10][152][153] Non-micronized estradiol tablets in doses of 0.125, 0.25, and 1 mg were previously marketed for use by sublingual administration under brand names such as Diogynets, Estradiol Membrettes, and Dimenformon in the 1950s.[154][155][156][157][158] Non-micronized estradiol has poor water solubility, but micronized estradiol is rapidly absorbed by the sublingual route.[152] All oral estradiol tablets are micronized, as this improves the efficiency of estradiol absorption in the gastrointestinal tract.[36] Likewise, all oral estradiol valerate tablets seem to be micronized.[44] The sublingual route is, in actuality, probably a combination of sublingual and oral delivery of estradiol due to incidental swallowing of some of the estradiol.[95]
The absorption of sublingual estradiol can be attributed to the rich vascularization under the tongue.[152] With administration of an oral estradiol tablet sublingually, complete dissolution of the tablet occurs within a few minutes and circulating levels of estradiol begin to rise within 5 minutes.[152] Maximal levels of estradiol occur after 30 to 60 minutes of administration.[152] After this, estradiol levels drop steeply within 4 hours, and this is followed by a more gradual decline in levels of estradiol and a return to baseline concentrations by 24 hours.[152] The rapid rise and steep fall of estradiol levels with sublingual administration of estradiol is analogous to the case of intravenous injection and intranasal administration of the hormone.[10][12][4]
Sublingual administration of medications that are subject to a high first-pass effect with oral administration can result in improved bioavailability because the first pass through the intestines and liver is bypassed.[152] As a result, sublingual estradiol has been found to result in estradiol levels and a ratio of estradiol to estrone that are substantially higher than oral estradiol.[10][152][159] Maximal circulating levels of estradiol are as much as 10-fold higher with sublingual administration than with oral administration, and the absolute bioavailability of estradiol is approximately 5-fold higher.[10][152] On the other hand, levels of estradiol fall rapidly with sublingual administration, whereas they remain elevated for a prolonged period of time with oral administration.[10][12] This is due to the large circulating pool of hormonally inert estrogen conjugates with long half-lives that is reversibly generated with oral estradiol during first-pass metabolism, which serves as a metabolism-resistant and long-lasting reservoir for continuous reconversion back into estradiol.[10][12] It is also responsible for the differences in ratios between sublingual estradiol and oral estradiol in terms of maximal estradiol levels (10:1) achieved and absolute bioavailability (5:1).[10][12] A study in marmoset monkeys found that the bioavailability of sublingual estradiol was 10% of that of estradiol administered by intramuscular injection.[3]
After a dose of sublingual estradiol, levels of estrone start to slowly but progressively rise within 10 minutes.[152] Estrone levels surpass estradiol levels at around 2 hours post-dose and reach a maximum at about 4 hours.[152] It has been speculated that the high delayed levels of estrone with sublingual estradiol may be due to the rich lymphatic drainage in the neck region, which may result in estradiol being taken up by the reticuloendothelial system and then metabolized into estrone.[152]
Sublingual administration of a single 0.25 mg tablet of micronized estradiol has been found to produce peak levels of 300 pg/mL estradiol and 60 pg/mL estrone within 1 hour.[10] A higher dose of 1 mg estradiol was found to result in maximum levels of 450 pg/mL estradiol and 165 pg/mL estrone, which was followed by a rapid decline in estradiol levels to 85 pg/mL within 3 hours.[10] Conversely, the decline in estrone levels was much slower and reached a level of 80 pg/mL after 18 hours.[10] A single administration of 4 mg micronized estradiol (two 2-mg Estrace tablets) under the tongue, considered a very high dose of sublingual estradiol, has been found to result in maximal levels of estradiol of 1759 ± 704 pg/mL, with a range of 634 to 2840 pg/mL, after 1 hour in a mixed group of normotensive and hypertensive postmenopausal women.[160] A replication of this study using the same dosage and protocols measured estradiol levels of 2227 ± 1180 pg/mL for the whole group of women but found that estradiol levels between the normotensive and hypertensive groups were significantly different at 1790 ± 869 pg/mL and 2664 ± 1490 pg/mL, respectively.[161][162]
Although sublingual administration of estradiol has a relatively short duration, the medication can be administered multiple times per day in divided doses to compensate for this.[10][163][164] Studies that used high doses of sublingual estradiol in the treatment of severe postpartum depression have administered a dose of 1 mg 3 to 8 times per day.[165][166][163][164] In one study, which administered a mean total dosage of sublingual estradiol of 4.8 mg/day, estradiol levels remained elevated at about 130 pg/mL on average in the morning before the first dose of the day.[165]
Oral micronized estradiol valerate tablets can be taken sublingually as well.[167][168] The administration of 2 mg oral micronized estradiol valerate tablets (Progynova, Schering) sublingually 3 or 4 times per day resulted in circulating estradiol levels of about 290 pg/mL to 460 pg/mL in premenopausal women (time of measurements not given).[167][168] Steady-state levels of estradiol were achieved within about 5 or 6 days.[167][168] Levels of progesterone, luteinizing hormone, and follicle-stimulating hormone were all considerably suppressed, and ovulation, as well as the associated mid-cycle hormonal surges, were prevented.[167][168] Sublingual estradiol valerate is used for cycle control in egg donation and surrogacy in cisgender women and is used in hormone therapy for transgender women.[167][168][169]
Cyclodextrin-containing formulations of sublingual estradiol with improved water solubility and absorption have been developed and studied.[170][171][172][173][174]
Clinical effects
[edit]The total endometrial proliferation dose of sublingual estradiol in women is 60 to 140 mg per cycle or 14 days and of sublingual estradiol benzoate in women is 60 to 180 mg per cycle or 14 days.[75]: 310 Both sublingual estradiol and sublingual estradiol benzoate have a persistence of estrogenic effect after a dose of only one day.[75]: 310 The effects of sublingual estradiol on gonadotropin levels have also been studied in postmenopausal women.[152][175][153][176] After a dose of sublingual estradiol, levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decrease precipitously within 4 hours.[152] Following this, LH and FSH levels gradually increase, and return to near-baseline levels by 24 hours.[152] One study found no difference between oral and sublingual estradiol in suppression of LH levels.[152] However, FSH levels were suppressed to a greater extent with sublingual estradiol than with oral estradiol in the study.[152]
It is notable that the magnitude of the genomic effects of estradiol (i.e., signaling through the nuclear ERs) may, at least in some cases, be dependent on the total estrogenic exposure as opposed to the duration of exposure.[10] For instance, in normal human epithelial breast cells and ER-positive breast cancer cells, the rate of breast cell proliferation has been found not to differ with estradiol incubation of 1 nM for 24 hours and incubation of 24 nM for 1 hour.[10] In other words, short-term high concentrations and long-term low concentrations of estradiol appear to have the same degree of effect in terms of genomic estrogenic signaling, at least in terms of breast cell proliferation over a 24-hour period.[10] On the other hand, non-genomic actions of estradiol, such as signaling through membrane estrogen receptors like the GPER, may be reduced with short-term high concentrations of estradiol relative to more sustained levels.[10] For instance, although daily intranasal administration of estradiol is associated with comparable clinical effectiveness (e.g., for hot flashes) relative to longer acting routes of estradiol administration in postmenopausal women, it is also associated with significantly lower rates of breast tension (tenderness and enlargement) relative to longer acting estradiol routes, and this is thought to reflect comparatively diminished non-genomic signaling.[10]
Graphs
[edit]-
Estradiol levels over a 24-hour period following a single 0.25, 0.5, or 1 mg dose of sublingual estradiol or a single 0.5 or 1 mg dose of oral estradiol in postmenopausal women. Source: Price et al. (1997).
-
Hormone levels after a single 0.5 mg dose of sublingual estradiol in postmenopausal women. Source: Burnier et al. (1981).
-
Hormone levels after a single 2 mg dose of sublingual estradiol in premenopausal women. Source: Casper & Yen (1981).
-
Hormone levels after a single 0.5 mg dose of sublingual estradiol in postmenopausal women. Source: Fiet et al. (1982).
-
Estradiol levels with 2 to 12 mg/day sublingual estradiol taken along with spironolactone in transgender women. Error bars are SEM. Time of blood collection and time and frequency of administration were not specified. Source: Jain et al. (2019).
-
Hormone levels with 2 mg oral micronized estradiol valerate tablets (Progynova, Schering) taken 3 or 4 times per day (6–8 mg/day total) sublingually (SL) in premenopausal women. Time of blood collection after medication administration was not specified. Sources: Serhal et al. (1989, 1990).
-
Trough estradiol levels and MADRS scores with 1 mg sublingual micronized estradiol 3 to 8 times per day (3 to 8 mg/day total; mean 4.8 mg/day total) in women with postpartum depression. Blood was drawn specifically in the mornings before the first dose of sublingual estradiol for the day. Source: Akohas et al. (2001).
Intranasal administration
[edit]
Estradiol has been studied and used by intranasal administration.[72][10] It was available as a cyclodextrin-containing nasal spray under the brand name Aerodiol in some countries,[178][179][180][181] although this specific product was discontinued in 2007.[182][183] The product was administered once per day as one 150-μg spray in each nostril (300 μg/day total).[184] Intranasal estradiol has pharmacokinetics similar to those of sublingual and intravenous administration of estradiol, including a sharp peak and then rapid decline in estradiol levels.[10] Despite the relatively short duration of intranasal estradiol, it has similar effectiveness to other, longer-lasting routes of administration in terms of relief of menopausal symptoms like hot flashes.[10]
Transdermal administration
[edit]Transdermal estradiol is available in the forms of patches, gels, emulsions, and sprays.[185][186][10][17][187] In the case of gels, emulsions, and sprays, the route is sometimes referred to as topical rather than as transdermal.[186][188][5] Topical administration can also refer to vaginal administration of gels and creams however. [citation needed]
Estradiol has moderate skin permeability, which is based on the lipophilicity and hydrophilicity of a compound.[10][189] In general, the more polar groups, such as hydroxyl groups, that are present in a steroid, and hence the more hydrophilic and less lipophilic it is, the lower its skin permeability.[10][189] For this reason, estrone and progesterone have higher skin permeability, while estriol and cortisol have lower skin permeability.[10] The transdermal bioavailability of estradiol in an alcohol solution is approximately 10%.[190][189] Transdermal estradiol reservoir patches have been reported to have a bioavailability of 3 to 5%.[191] Estradiol is a highly potent compound and circulates at picomolar concentrations (pg/mL), which makes it ideal for transdermal application as only small amounts of substance need to be delivered across the skin.[96] Conversely, progesterone, which circulates at levels in the nanomolar range and requires a far higher quantity of substance for biological effect, is not well-suited for transdermal delivery.[96] Fatty acid esters of estradiol, such as estradiol benzoate, estradiol valerate, and estradiol cypionate, have been found to have similar estrogenic potency to estradiol but a comparatively longer duration with transdermal administration in animal studies.[192][193]
Regardless of administration form, such as patch or gel, transdermal estradiol is transported into the skin, including through the stratum corneum, epidermis, and dermis, by a passive diffusion process.[10][194] Following this, estradiol is then taken up by local capillary blood vessels and delivered into the circulation.[10] There is a depot effect in the skin with transdermal estradiol, which results in continuous delivery of transdermal estradiol into the circulation.[17][194] This is because the skin functions as a semipermeable membrane and there is a concentration gradient between the application site of transdermal estradiol and capillary blood, with the rate of diffusion of estradiol across the stratum corneum being the specific rate-limiting factor in absorption.[10][194] As a result, peaks and troughs in circulating estradiol levels are limited, and the skin and subcutaneous fat act as a reservoir of estradiol that maintains circulating estradiol levels between doses.[17] For these reasons, transdermal estradiol can provide near-constant circulating levels of estradiol, similarly to oral estradiol.[17][10] Enzymes that metabolize estradiol are minimally expressed in the skin, and for this reason, the metabolism of estradiol in the skin is low.[10]
The site of application of transdermal estradiol can influence its bioavailability.[96] A study found comparable absorption of transdermal estradiol patches (within ±25% of reference) for a number of skin sites including the abdomen, upper arm, upper thigh, lower back, and side.[195][196] However, absorption was 15% lower for the upper thigh compared to the abdomen and the difference was significant.[197][196] Another study found that transdermal estradiol patches had 20 to 25% higher bioavailability when applied to the buttocks than when applied to the abdomen.[96] Studies of topical steroids have found that the scrotum is especially permeable among skin sites.[198] Studies of transdermal testosterone cream, gel, and patches applied to the scrotum in men have observed 5- to 8-fold higher levels of testosterone than with application to conventional skin sites.[199][200] In a study of topical application of hydrocortisone solution in men, skin permeability (defined as total radiolabeled urinary excretion) relative to the forearm (1.0) was 42.0 for the scrotum, 13.0 for the jaw angle, 6.0 for the forehead, 3.6 for the underarm, 3.5 for the scalp, 1.7 for the back, 0.8 for the palm of the hand, 0.4 for the ankle, and 0.1 for the sole of the foot.[198][201][202][203] In accordance with findings with other topical steroids, a study in men with prostate cancer treated with transdermal estradiol patches applied to the scrotum observed about 5-fold higher estradiol levels relative to application to conventional skin sites such as the forearm.[204][205] Penile skin may have similarly enhanced absorption characteristics relative to scrotal skin.[206]
Transdermal estradiol bypasses the intestines and liver and hence the first-pass metabolism that is associated with oral administration.[10][96] In addition, unlike oral estradiol, transdermal estradiol is not associated with supraphysiological concentrations of estrone or estrogen conjugates like estradiol sulfate, and transdermal estradiol does not have disproportionate effects on liver protein synthesis.[10][96] In accordance, estradiol, at typical menopausal replacement dosages, has been found not to increase the risk of blood clots or insulin resistance,[118][12] nor to affect hepatic SHBG, IGF-1, GHBP,[119] IGFBP,[120] and other protein production and by extension circulating hepatic protein levels.[122][123][121][96] However, at higher doses, transdermal estradiol has been associated with a significantly higher incidence of stroke in postmenopausal women, probably due to blood clots.[207][208] Another larger study did not find a significantly higher risk of blood clots with similar doses of transdermal estradiol however.[209]
Transdermal patches
[edit]
Estradiol patches have an extended duration and are available for twice-weekly (3–4-day) and once-weekly (7-day) application, while gels, emulsions, and sprays are administered daily.[186][15][10][210] There are two types of estradiol patches: reservoir patches, which have been described as first-generation patches, and matrix patches, which are considered to be improved second-generation patches.[10][12][186] Reservoir patches were designed for twice-weekly application, while matrix patches have been produced for both twice-weekly and once-weekly application.[12] Reservoir patches of estradiol (e.g., Estraderm) are mostly no longer used, with most estradiol patches available today being matrix patches (e.g., Alora, Climara, Esclim, Estradot, FemPatch, Menostar, Oesclim, Vivelle, and Vivelle-Dot).[186]
| Brand name | Dose (µg/day) |
DOA (d) | Size[b][c] (cm2) |
Levels (pg/mL) |
Intro. |
|---|---|---|---|---|---|
| Alora | 25, 50, 75, 100 | 3–4 | 9, 18, 27, 36 | 43–144 | 1996 |
| Climara[d] | 25, 37.5, 50, 60, 75, 100 |
7 | 6.5, 9.375, 12.5, 15, 18.75, 25 |
17–174 | 1994 |
| Climara Pro[e] | E2 (45) LNG (15) |
7 | 22 | 27–54 | 2003 |
| CombiPatch[e] | E2 (50) NETA (14, 25) |
3–4 | 9, 16 | 27–71 | 1998 |
| Menostar | 14 | 7 | 3.25 | 13–21 | 2004 |
| Minivelle | 25, 37.5, 50, 75, 100 |
3–4 | 1.65, 2.48, 3.3, 4.95, 6.6 |
30–117 | 2012 |
| Vivelle | 50, 100 | 3–4 | 14.5, 29 | 30–145 | 2000 |
| Vivelle-Dot[d] | 25, 37.5, 50, 75, 100 |
3–4 | 2.5, 3.75, 5, 7.5, 10 |
30–145 | 1996 |
A dosage of 1 mg/day oral estradiol is considered to be roughly equivalent to 25 or 50 μg/day transdermal estradiol and a dosage of 2 mg/day oral estradiol is considered to be equivalent to 50 or 100 μg/day transdermal estradiol depending on the source.[213][97][12][10] Estradiol patches delivering a daily dosage of 0.05 mg (50 μg) achieve mean estradiol and estrone levels of 30 to 65 pg/mL and 40 to 45 pg/mL, respectively, while a daily dosage of 0.1 mg (100 μg) attains respective mean levels of 50 to 90 pg/mL and 30 to 65 pg/mL of estradiol and estrone.[15] In general, Climara-type estradiol transdermal patches have an approximate 1:1 ratio of estradiol delivered in μg/day relative to circulating estradiol concentration in pg/mL.[205] In other words, a 100 μg/day Climara estradiol patch may be expected to produce circulating estradiol levels of around 100 pg/mL.[205] Transdermal estradiol patches produce an estradiol to estrone ratio of about 1:1.[10][12] Following removal of an estradiol patch, circulating estradiol levels decrease to baseline within 24 hours.[10]
Typical dosages of estradiol patches are intended to provide the minimum amount of estrogen replacement necessary for the effective alleviation of menopausal symptoms, and for this reason, they achieve relatively low levels of estradiol.[10] A dosage of two to six 100 μg/day transdermal estradiol patches can achieve mean levels of estradiol in the area of 200 to 400 pg/mL and can be used as a form of high-dose estrogen therapy, for instance to suppress testosterone levels in the treatment of prostate cancer in men and in feminizing hormone therapy for transgender women.[14][214][215] High-dose transdermal estradiol patches have also been studied in the treatment of postpartum depression and postpartum psychosis; in one such study, 200, 400, and 800 μg/day estradiol in the form of transdermal patches resulted in estradiol levels of 286 pg/mL, 675 pg/mL, and 1032 pg/mL, respectively.[216] In another study, estradiol levels with 800 μg/day estradiol in the form of transdermal patches (Estraderm TTS) resulted in estradiol levels of 690 to 815 pg/mL.[217] However, there is erratic absorption and considerable variation in estradiol levels using high-dose estradiol patches both between and within individuals, with one study finding that estradiol levels ranged from 70 pg/mL to 1,045 pg/mL (mean 460.7 pg/mL) with six 100 μg/day estradiol patches.[218][219]
The Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) study is a randomized controlled trial of high-dose transdermal estradiol patches versus gonadotropin-releasing hormone agonist monotherapy in the treatment of prostate cancer in approximately 2,200 men.[220][221][222] It is specifically comparing three to four 100 μg/day estradiol patches (FemSeven) against goserelin implants.[220] The study was started in March 2006 and is estimated for completion in August 2021.[220] Its objectives include comparison of survival, cardiovascular mortality and morbidity, pharmacological activity (e.g., suppression of testosterone levels), other side effects and toxicities, and quality of life.[220] In addition to the PATCH trial, the Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) study added a high-dose estradiol patches arm (~2,000 men) in July 2017.[223][221][222]
Estradiol patches are associated with local skin reactions and such as irritation in 14.2% of individuals (with reservoir patches), mild-to-moderate erythema (redness) in 50 to 60% of individuals, and allergic reactions due to cutaneous sensitization.[10][12] Up to 5% of people using reservoir patches may discontinue therapy due to skin reactions.[12] Visible adhesive residues are also often left by estradiol patches following their removal.[10] Transdermal estradiol gel can serve as an alternative to transdermal estradiol patches for individuals who experience intolerable skin reactions with them.[224] Estradiol patches should not be applied to the breast as this may result in high local levels of estradiol in the breasts and hence an increased likelihood of breast tenderness.[225]
-
Levels of estradiol over a period of 8 days after a single application of a 50 or 100 μg/day Climara-type (Climara, Menostar, Mylan generic) once-weekly transdermal estradiol matrix patch to the abdomen and removed on day 7 in postmenopausal women.[232]
-
Levels of estradiol and estrone with application of a single 50 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[188]
-
Estradiol level with a single 100 µg/day estradiol reservoir patch (Estraderm) with and without ethanol added in postmenopausal women.[17][233] This patch has a 3- to 4-day duration and is designed for twice-weekly application. In one group, ethanol was injected into the area between the patch and the skin on day 3.[17][233] This gave significantly higher and prolonged estradiol levels.[17][233]
-
Estradiol levels with 50 to 100 μg/day transdermal estradiol patches applied to the forearm and to the scrotum in a crossover study in 2 men with prostate cancer.[204] In 35 men treated continuously with one 100 μg/day estradiol patch scrotally, the mean estradiol level was ~500 pg/mL (range ~125–1,200 pg/mL).[204]
Transdermal gel
[edit]Estradiol is available as a transdermal gel in the form of gel dispensers and gel packets. Major estradiol gel dispenser products include EstroGel and Elestrin while major estradiol gel packet products include DiviGel and Sandrena. Estradiol gels are administered daily.[186][15][10][212] When estradiol is administered as a hydroalcoholic gel, it dries within 2 to 5 minutes following application to the skin.[194] A single application of a transdermal estradiol gel results in a sustained increase in estradiol levels for at least 24 hours.[17][194] The apparent elimination half-life of estradiol with transdermal estradiol gel is 36 hours.[194]
Once daily application of 1.25 g topical gel containing 0.75 mg estradiol (brand name EstroGel) for 2 weeks was found to produce mean peak estradiol and estrone levels of 46.4 pg/mL and 64.2 pg/mL, respectively.[194] The time-averaged levels of circulating estradiol and estrone with this formulation over the 24-hour dose interval were 28.3 pg/mL and 48.6 pg/mL, respectively.[194] Levels of estradiol and estrone are stable and change relatively little over the course of the 24 hours following an application, indicating a long duration of action of this route.[194] Steady-state levels of estradiol are achieved after 3 days of application.[194] A higher dosage of estradiol gel containing 1.5 mg estradiol per daily application has been found to produce mean estradiol levels of 40 to 100 pg/mL and estrone levels of 90 pg/mL, while 3 mg per day has been found to result in respective mean estradiol and estrone levels of 60 to 140 pg/mL and 45 to 155 pg/mL.[15] Topical estradiol gel at a dosage of 3 mg/day has been reported to be equipotent with 2 mg oral estradiol in terms of therapeutic effects and FSH suppression, as well as to produce similar estradiol levels.[129] Transdermal estradiol gel produces an estradiol to estrone ratio of about 1:1.[10][12]
Transdermal estradiol gel can be used as a form of high-dose estrogen in transgender women.[224] However, the doses needed require application to a large surface of skin that amounts to the combined area of both legs for proper absorption.[224] As a result, high-dose transdermal estradiol gel is not a primary choice of estrogen therapy for most transgender individuals.[224] Similarly to transdermal estradiol patches, high-dose transdermal estradiol gel has been studied in the treatment of prostate cancer as well.[235][236][237][238][239][240][241] In these studies, levels of estradiol with estradiol gel or ointment were 84 pg/mL with 3 mg/day, 185 pg/mL with 6 mg/day, 107 pg/mL with 10 mg/day, and 473 pg/mL with 20 mg/day.[236][237][238][239][240][241] In women, high doses of estradiol gel, including 3 mg/day, 4 mg/day, and 8 mg/day, have been reported to produce estradiol levels of 99 pg/mL, 117 pg/mL, and 204 pg/mL, respectively.[242][70]
Studies have found that topical application of estradiol to the breasts increases local levels of estradiol in breast tissue.[243][244][245][246]
The total endometrial proliferation dose of transdermal estradiol gel in women has been reported to be 150 mg per cycle or 14 days.[247][75]: 310 However, it has also been found that 6 mg/day estradiol gel is effective for endometrial proliferation in women.[248]
-
Levels of estradiol and estrone with once daily application of 1.25 g of a transdermal estradiol gel (EstroGel) containing 0.06% or 0.75 mg estradiol after 14 days of continuous therapy in postmenopausal women.[194]
-
Estradiol levels after the last dose with 1 mg/day transdermal estradiol gel applied to different amounts of skin area (200 cm2, 400 cm2, or as large as possible) in postmenopausal women.[250]
Other transdermal formulations
[edit]

Estradiol is available in the form of transdermal emulsions (e.g., Estrasorb) and sprays (e.g., Lenzetto, Evamist).[187] Estradiol emulsions and sprays are administered daily.[186][15][10][212] The pharmacokinetics of these preparations have been studied.[251][252][253]
Variability in pharmacokinetics
[edit]Transdermal estradiol patches are described as delivering a fixed amount of estradiol such as 50 μg/day or 100 μg/day.[10] However, there is large interindividual variability and intraindividual variability in the pharmacokinetic parameters of transdermal estradiol, and fluctuations in circulating estradiol levels with estradiol patches are almost as great as with oral estradiol.[10][96][12][17] As such, the actual delivery rate of estradiol and mean levels of estradiol achieved with transdermal estradiol patches may be different from what is described and from the mean levels observed in clinical studies, respectively.[10]
A wide range of estradiol levels are measured in women using the same estradiol patch or gel and dosage, with an up to about 10-fold difference in levels.[10][96][17] In a study of estradiol gel and patches, the maximal difference in peak levels between individuals was 11-fold for the gel and 7-fold for the patch, and the maximal difference in area-under-the-curve levels (total exposure) was 6-fold for the gel and 8-fold for the patch.[96] It has likewise been reported that the interindividual variability in bioavailability with Estraderm reservoir patches ranges from 25 to 225%.[17] In as many as 30% of women treated with a 50 μg/day estradiol patch, estradiol levels are low.[10] There are also significant short-term intraindividual differences in estradiol levels with estradiol patches; estradiol levels can fluctuate considerably from hour to hour.[10][188] In addition, estradiol levels with estradiol patches are higher in the evening than in the morning, which may be due to circadian variations in skin blood flow that may influence absorption.[10] In terms of area-under-the-curve levels of estradiol, the interindividual variability of transdermal estradiol has been found to be 20 to 44% using different transdermal formulations, and the intraindividual variability with transdermal estradiol has been found to be 20%.[12]
Factors which may contribute to inter- and intraindividual variability with transdermal estradiol include skin location and thickness; hair follicle density; solvent (alcohol) evaporation; skin dehydration, ambient temperature, and humidity; and reservoir size.[17]
Vaginal administration
[edit]Vaginal estradiol is available in the forms of tablets, creams, inserts or suppositories, and rings.[186][10][185] Vaginal estradiol tablets, creams, and inserts are usually administered once daily to twice weekly, whereas vaginal estradiol rings have a sustained action and are replaced once every 90 days.[186][10] Vaginal estradiol can be used both as a systemic form of estradiol therapy, and at very low doses to selectively achieve a local vaginal effect without systemic effects, for instance in the treatment of menopausal symptoms such as vaginal atrophy and dryness.[10][254]
Vaginal estradiol is rapidly and almost completely absorbed.[72] The absorption of vaginal estradiol is slightly greater in women with vaginal atrophy.[72] Vaginal estradiol has high bioavailability and greatly increased potency compared to oral estradiol, with about 10- to 20-fold the comparative potency of oral estradiol.[10] The greater potency of vaginal estradiol relative to oral estradiol is due to the lack of the first pass associated with oral estradiol and due to low local metabolism of estradiol in the vagina.[10] Because of the high estradiol levels achieved, LH and FSH are more strongly suppressed with vaginal estradiol than with other routes.[72]
A daily dosage of 0.5 mg vaginal micronized estradiol has been found to result in estradiol and estrone levels of 250 pg/mL and 130 pg/mL, respectively.[15] Vaginal estradiol has a much higher estradiol-to-estrone ratio in comparison to oral estradiol.[10] The average ratio of estradiol to estrone with vaginal estradiol is 5:1, compared to 1:5 in the case of oral estradiol, a 10-fold difference.[10]
As vaginal estradiol is not subject to a first pass and bypasses the intestines and liver, it does not affect liver protein synthesis at menopausal replacement dosages, similarly to transdermal estradiol.[255] On the other hand, a first pass effect in the uterus may occur with vaginal administration of estradiol and this may have implications for uterine safety.[256]
Rectal administration
[edit]
Estradiol has been assessed for use by rectal administration in a number of studies.[242][70][261][262][263] Uses of estradiol by this route have included treatment of menopausal symptoms in postmenopausal women.[242][70][261][262] Rectal administration of estradiol is described as qualitatively and quantitatively similar to vaginal administration of estradiol.[261][262][264] The use of estradiol by the rectal route considerably bypasses the liver and hence the first-pass metabolism that occurs with oral estradiol, similarly to other parenteral routes of estradiol such as vaginal and transdermal administration.[242][265] Irritation of the intestines does not usually occur with rectal estradiol.[261] The use of estradiol by the rectal route is not well-accepted by all individuals,[261] and due to its inconvenience, it has been said that rectal administration of estradiol has gained no practical clinical importance.[265]
Lauritzen (1986) reported that 3 hours after a single rectal dose of 1 mg micronized estradiol, estradiol levels increased by 620 pg/mL and estrone levels increased by 120 pg/mL.[261][72] Subsequently, Lauritzen (1987, 1990) reported that 0.5 mg/day and 1 mg/day rectal estradiol resulted in respective estradiol levels of 363 pg/mL and 515 pg/mL 6 hours following the last dose.[242][70] These estradiol levels are fairly similar to those achieved by vaginal estradiol.[261][70][72] The estradiol-to-estrone ratio of rectal estradiol is about 5:1, which likewise is the same as that of vaginal estradiol.[242][261][72] Absorption of rectal estradiol occurs rapidly within 30 to 60 minutes, maximal estradiol levels occur at 3 hours post-dose, and circulating estradiol levels are reportedly maintained for 4 to 10 hours.[261][262][72] The duration of rectal estradiol is said to necessitate repeated administration 1 to 2 times per day.[261][262]
Rectal administration of estriol, which has similar properties to estradiol, has also been studied.[266] Administration of a rectal suppository containing 100 mg estriol resulted in estriol levels in pregnant women at term increasing by about 53%.[266] Estriol levels at term are normally between 5,000 and 20,000 pg/mL, which suggests that estriol levels may have increased following the suppository by about 5,000 to 10,000 pg/mL (precise levels were not provided).[267][268][269]
Intramuscular injection
[edit]Intramuscular injections are injections into muscle, for instance the gluteal or deltoid muscle. Estradiol and estradiol esters can be administered in a variety of forms by intramuscular injection.[270][10][271] Aqueous solutions of estradiol and estradiol esters by intramuscular injection have a rapid onset and duration analogously to but slightly more delayed than intravenous injection.[citation needed] However, intramuscular injections of oil solutions, crystalline aqueous suspensions, and emulsions of estradiol and estradiol esters, as well as solutions and suspensions of estradiol polymers and estradiol microspheres, act as long-lasting depot injections.[citation needed]
Estradiol esters, including but not limited to estradiol benzoate, estradiol valerate, estradiol cypionate, estradiol enanthate, and estradiol undecylate, are inactive prodrugs of estradiol that are converted into estradiol in the body.[10][272] The aforementioned estradiol esters are fatty acid esters and are more lipophilic (fat-soluble) than estradiol.[citation needed] More lipophilic compounds are absorbed more slowly from the injection site when given by depot intramuscular injection (as oil solutions, aqueous suspensions, and emulsions), and hence more lipophilic estradiol esters have longer durations than free estradiol or less lipophilic estradiol esters via this route.[citation needed] Polyestradiol phosphate is a polymer of the hydrophilic (water-soluble) estradiol ester estradiol phosphate which circulates in the blood but is metabolized into estradiol very slowly.[citation needed]
The bioavailability of estradiol and estradiol esters given by intramuscular injection is said to be essentially complete.[4] For comparison, the bioavailability of oral estradiol is around 5%.[10] The estradiol levels that result with typical clinical doses of estradiol and estradiol esters by intramuscular injection tend to be high compared to the typical estradiol levels that occur with other clinically used routes and forms of estradiol.[10][16][273][274][13]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Aqueous solutions
[edit]Aqueous solutions are solutions of a compound with water.[citation needed] In contrast to other formulations, such as oil solutions, aqueous suspensions, and emulsions, aqueous solutions of estradiol and estradiol esters by intramuscular injection are not depot injections.[citation needed] Instead, they are rapidly absorbed and eliminated, analogously to intravenous injections of estradiol and estradiol esters.[citation needed] The durations of aqueous solutions of estradiol and estradiol esters by intramuscular injection are measured in hours.[citation needed]
Oil solutions
[edit]Oil solutions are solutions of a compound with oil, for instance sesame oil or castor oil.[citation needed] When free steroids like estradiol are administered in oil solution by intramuscular injection, they are rapidly absorbed and the duration is relatively short.[270][275] A single 1 to 2 mg dose of estradiol in oil solution by intramuscular injection has a duration of about 1 or 2 days.[265][276][277] Little prolongation of duration is achieved with the use of larger doses.[270][278][279] Nonetheless, the duration of estradiol in oil solution by intramuscular injection is significantly longer than an intravenous injection of estradiol or estradiol valerate, which show a duration of only a few hours.[10][12][4][60][280][281]
Conversely, intramuscular injections of estradiol esters in oil solution have durations of days to months, depending on the ester administered.[265] Following a single 4 or 5 mg intramuscular injection in oil solution, peak estradiol levels are about 950 pg/mL with estradiol benzoate after 2 days, 400 to 650 pg/mL with estradiol valerate after 2 days, and 250 to 350 pg/mL with estradiol cypionate after 4 days.[274][16][273] The durations with a 5 mg dose are 4 or 5 days with estradiol benzoate, 7 or 8 days with estradiol valerate, and 11 to 14 days with estradiol cypionate.[274][16][273] The differences in estradiol levels and the different durations with estradiol levels are due to their different rates of release from the oily depot at the injection site.[274] The longer and hence more lipophilic the fatty acid ester, the slower the release from the depot, the lower the peak estradiol levels, and the longer the duration.[274][10][265]
The duration of estradiol esters in oil solution by intramuscular injection is dose-dependent.[282] With estradiol valerate, it is reported that a dose of 5 mg has a duration of 7 to 8 days,[274] 10 mg a duration of 10 to 14 days,[265][282] 40 mg a duration of 2 to 3 weeks, and 100 mg a duration of 3 to 4 weeks.[282] High doses of estradiol valerate, such as 40 mg per week, can achieve pregnancy levels of estradiol.[283] A study of pseudopregnancy with intramuscular injections of 40 mg/week estradiol valerate and 250 mg/week hydroxyprogesterone caproate observed estradiol levels of about 2,500 to 3,000 pg/mL.[283]
| Estrogen | Dose | Cmax | Tmax | Duration |
|---|---|---|---|---|
| Estradiol benzoate | 5 mg | E2: 940 pg/mL E1: 343 pg/mL |
E2: 1.8 days E1: 2.4 days |
4–5 days |
| Estradiol valerate | 5 mg | E2: 667 pg/mL E1: 324 pg/mL |
E2: 2.2 days E1: 2.7 days |
7–8 days |
| Estradiol cypionate | 5 mg | E2: 338 pg/mL E1: 145 pg/mL |
E2: 3.9 days E1: 5.1 days |
11 days |
| Notes: All via i.m. injection of oil solution. Determinations via radioimmunoassay with chromatographic separation. Sources: See template. | ||||
Aqueous suspensions
[edit]Aqueous suspensions are suspensions of crystal particles of a compound in water.[citation needed] Estradiol in microcrystalline aqueous suspension for use by intramuscular injection was previously marketed in the 1950s under brand names such as Aquadiol, Diogyn, Progynon Aqueous Suspension, and Progynon Micropellets.[284][285][286][287][288][289][290][291] It was used at a dose of 0.5 to 1.5 mg 2 or 3 times per week.[290] Newman (1950) found that 0.5 to 2 mg once per week was satisfactory.[292] As such, the preparation presumably had a duration in the range of 2 to 7 days.[290][292]
Microcrystalline aqueous suspensions of estradiol esters, for instance of estradiol benzoate (brand names Agofollin Depot alone and Follivirin in combination with testosterone isobutyrate),[293][294] have been found to have longer duration of actions than oil solutions of the same esters when administered via intramuscular injection.[295][296][271][297][298][49][299]: 310 Whereas the duration of a single intramuscular injection of amorphous estradiol benzoate in oil solution is 6 days, the duration of a single intramuscular injection of microcrystalline estradiol benzoate in aqueous suspension is 16 to 21 days.[75][296][300][301]
The duration of crystalline aqueous suspensions is highly dependent on crystal size.[302][303][298][304][305] Steroids and steroid fatty acid esters are lipophilic and have very low water solubility.[306] When they are suspended in the form of crystals in water, these crystals dissolve slowly, releasing steroid from their surfaces in the process.[306][307] The larger the particle sizes of the crystals, the slower the dissolution rate.[306] When a crystalline aqueous suspension of steroid is administered via intramuscular injection, a crystalline depot suspended in fluid is formed locally within the muscle.[306][307] These crystals slowly dissolve and the steroid is gradually absorbed into the body, resulting in the long durations of such preparations.[306][307] Particle sizes of 10 μm or less have no apparent depot effect.[308]
A larger needle size is needed for aqueous suspensions of steroids to allow the steroid crystals to pass through the needle lumen.[309][310] Aqueous suspensions pose a risk of injection site reactions such as local irritation, swelling, and redness, with often severe pain.[303][310] The reactions are worse with larger crystal sizes.[303][311] Particle sizes of more than 300 μm in the case of estradiol benzoate have been found to be too painful for use.[311] The local injection site reactions, which do not occur with oil solutions, have limited the clinical use of aqueous suspensions of estradiol and its esters as well as other steroids.[312][313][314]
Emulsions
[edit]Emulsions are mixtures of immiscible liquids. Water-in-oil emulsions of estradiol benzoate were evaluated as long-acting preparations for use by intramuscular injection in the 1940s and 1950s.[298][271] Formulations of estradiol benzoate alone under the brand name Menformon-Emulsion and with progesterone under the brand name Di-Pro-Emulsion were previously marketed.[315][316] A 10 mg dose of estradiol benzoate in emulsion by intramuscular injection is said to have a duration of about 2 to 3 weeks.[315] This is similar to the duration of an aqueous suspension of 10 mg estradiol benzoate or an oil solution of 10 mg estradiol valerate.[315] Emulsions of steroids by intramuscular injection have similar properties (e.g., duration) relative to aqueous suspensions.[298][271] Painful injection site reactions have been reported with emulsions similarly to suspensions.[317]
Polymers
[edit]Polymers are large molecules of repeating subunits. Polyestradiol phosphate (brand name Estradurin) is a water-soluble estradiol ester in the form of a polymer and a very slowly hydrolyzed prodrug of estradiol.[318][319] It is formulated as an aqueous solution and is given by intramuscular injection.[318][319] The medication has an exceptionally long duration of action, with an elimination half-life of about 70 days or 10 weeks following a single injection.[320] Estradiol levels during polyestradiol phosphate therapy are very constant and uniform.[320] Levels of estradiol after 6 months of treatment with polyestradiol phosphate were about 350, 450, and 650 pg/mL with doses of 160, 240, and 320 mg once per month, respectively.[13] Polyestradiol phosphate has mostly been discontinued and remains available only in a few countries.[319][321]
Microspheres
[edit]Microspheres are microscopic spherical particles which can be used to encapsulate compounds.[citation needed] Estradiol is available in the form of an aqueous suspension of 1.0 mg estradiol in microspheres for use by intramuscular injection once a month under the brand name Juvenum E in Mexico.[322][323] It achieves circulating estradiol levels of 163 pg/mL to 219 pg/mL in the first 3 to 12 hours following injection, which decrease to 42 to 66 pg/mL during the first 4 days post-injection and to 20 to 35 pg/mL after 8 days, with levels remaining in this range thereafter over 30 days.[322] These estradiol levels are similar to the normal levels that occur during the early follicular phase of the menstrual cycle in premenopausal women (24 to 75 pg/mL).[322] The elimination of the formulation follows three phases: a rapid phase in the first 2 days, a second phase during days 2 to 12 days with a biological half-life of 7 to 10 days, and a third phase in which estradiol levels remain elevated above baseline for up to 30 days.[322]
Graphs
[edit]-
Tritiated estradiol radioactivity in blood with a single intramuscular injection of 1.5 to 2.8 μg tritiated estradiol in aqueous solution in four women.[324] Peak blood radioactivity occurred within 15 minutes in three of the women and in the remaining woman after 2 hours.[324] Source: Davis et al. (1963).[324]
-
Estradiol levels after a single intramuscular injection of 5 mg estradiol benzoate, 5 mg estradiol valerate, or 5 mg estradiol cypionate in oil solution in women.[274] Source: Oriowo et al. (1980).[274]
-
Simplified curves of estradiol levels after intramuscular injection of different 5 mg estradiol benzoate, 5 mg estradiol valerate, 5 mg estradiol cypionate, or 10 mg estradiol enanthate in oil solution in women.[326] Source: Garza-Flores (1994).[326]
-
Vaginal cornification with a single intramuscular injection of 5 mg estradiol benzoate, 5 mg estradiol dipropionate, or 5 to 25 mg estradiol cypionate in oil solution in women.[327] Source: Schwartz & Soule (1955).[327]
-
Estradiol levels after a short intravenous infusion of 20 mg estradiol in aqueous solution or an intramuscular injection of an equimolar dose of estradiol benzoate, estradiol valerate, or estradiol undecylate in oil solution in women.[328][280] Sources: Geppert (1975) and Leyendecker et al. (1975).[328][280]
-
Estradiol levels after a single intramuscular injection of 10 mg estradiol valerate or 100 mg estradiol undecylate in oil solution.[329] Source: Vermeulen (1975).[329]
-
Estradiol and testosterone levels after a single intramuscular injection of 320 mg polyestradiol phosphate in aqueous solution in men with prostate cancer.[320] Source: Stege et al. (1996).[320]
-
Estradiol levels after the fourth dose during continuous therapy with estradiol and progesterone microspheres in aqueous suspension by intramuscular injection once per month in menopausal women.[330][331] Source: Espino y Sosa et al. (2019).[330]
Subcutaneous injection
[edit]
Estradiol esters like estradiol valerate and estradiol cypionate can be given by subcutaneous injection instead of intramuscular injection.[332] Subcutaneous and intramuscular injection of estradiol cypionate in an aqueous suspension has been found to result in levels of estradiol and other pharmacokinetic parameters (e.g., duration) that were virtually identical.[8] Studies have shown that subcutaneous injection of closely related steroid esters in oil like the androgen esters testosterone cypionate, testosterone enantate, and nandrolone decanoate is effective and has similar pharmacokinetics to intramuscular injection as well.[333][215][334][335][336][337][338][339] In addition, studies have found that many intramuscular injections are really subcutaneous injections, as individuals often do not actually penetrate deep enough to inject into muscle when attempting to perform an intramuscular injection and instead inject into the subcutaneous fat layer above the muscle.[340][341] This is particularly prevalent with injections into the buttocks and in overweight and obese individuals, due to the thicker layer of fat over muscle.[340][341] Subcutaneous injections of estradiol esters may be easier and less painful to perform than intramuscular injections, and hence may result in improved compliance and satisfaction with therapy.[8]
Subcutaneous implantation
[edit]
Estradiol can be administered in a very long-lasting form via subcutaneous implantation of pure crystalline estradiol compressed into a small solid cylindrical pellet.[10][343] These pellets slowly and completely dissolve and are replaced once every 6 to 12 months, achieving high and very constant circulating levels of estradiol.[10][344][345] They are surgically inserted with the aid of a trocar by a trained physician in a medical office or clinic, and can be placed into locations including the lower abdomen, lower back, buttocks, or hips.[10][344][343] Subcutaneous pellets containing 20 mg estradiol (brand name Meno-Implant) or 25, 50, or 100 mg estradiol (brand name Estradiol Implants; discontinued) for replacement usually once every 6 months (range 4 to 8 months) are or have been available as approved pharmaceutical medications.[345] Up to 800 mg estradiol per implantation has been used.[346] Pharmaceutical estradiol pellet implants have been used almost exclusively in the United Kingdom, but have also been available in Australia and the Netherlands.[347][348] However, estradiol pellets have been discontinued in both the United Kingdom and Australia.[349][350] An estradiol implant has not been approved by the FDA as a pharmaceutical medication in the United States, but hormone pellet implants, including estradiol pellets, are available as custom compounded products in this country.[351][352][353]
Estradiol pellet implants are advantageous in that some women seem to need higher levels of estradiol for adequate relief of menopausal symptoms, and subcutaneous estradiol pellets are easily able to achieve such levels.[345][10] Conversely, this is not necessarily the case with oral or transdermal estradiol.[345][10] Another major advantage of estradiol pellet implants is convenience and guaranteed compliance.[345] They also do not have the issues pertaining to first-pass metabolism and liver protein synthesis of oral estradiol.[345][10] A major disadvantage of estradiol pellet implants is that they cannot be easily removed should this be necessary.[345] There are also concerns about accumulation of estradiol levels with long-term repeated pellet implantation.[345][10] Estradiol levels may remain above baseline for a year or in some cases 3 to 4 years following the last pellet insertion.[345] During this time, progestogen therapy should be continued to avoid the risk of endometrial changes.[345][344] Regular monitoring of estradiol levels and adjustment of dosing is recommended during therapy with estradiol pellet implants.[345]
Tachyphylaxis of relief of vasomotor symptoms, or hot flashes returning even with normal or supraphysiological estradiol levels, may occur in a small subset of cases with estradiol pellet implants.[345][10][347][354][344] The reason for this is unknown, but has been hypothesized to be a paradoxical effect of the high levels of estradiol achieved and/or a result of receptor desensitization caused by the long-term gradually decreasing levels of estradiol.[345][10] Such symptoms have been said to occur once estradiol levels begin to decrease, although there are also reports of such symptoms occurring 3 to 16 weeks (1 to 4 months) after pellet insertion, when estradiol levels should still be constant.[345][10] Hot flashes have notably been reported in pregnant women, who have very high and constantly increasing levels of estradiol.[355] When recurrence of hot flashes occurs with estradiol pellets, treated women often complain that their pellet has "run out".[345] Such symptoms can be temporarily offset with the use of supplemental oral or transdermal estradiol.[345]
Following insertion of an estradiol pellet, levels of estradiol rapidly increase, remain constant for about 4 months, and then gradually decrease.[345] A 25 mg subcutaneous estradiol pellet has been found to result in average estradiol levels of 90 pg/mL for 6 months, while two 25 mg pellets (50 mg total) resulted in estradiol levels of 180 pg/mL after 24 hours and levels of 100 to 120 pg/mL for 6 months.[10] Higher-dose pellets resulted in estradiol levels for 50 mg of 100 pg/mL, for 75 mg of 140 pg/mL, and for 100 mg of 150 pg/mL.[10] Estradiol levels are generally 50% higher than those of estrone, for an estradiol-to-estrone ratio of 1.5:1.[10] Very high levels of estradiol of between 400 and 1,000 pg/mL have been observed in a small subset of women treated with estradiol pellets and notably in those experiencing symptoms of tachyphylaxis.[345][10]
Estradiol pellet implants have been studied in the treatment of prostate cancer in men.[356][357][358][359][360]
Intrauterine administration
[edit]Intrauterine estradiol has been studied in the treatment of uterine hypoplasia in women.[361][362][297]
Intravenous injection
[edit]The administration of estradiol by intravenous injection has been studied.[60][280][363][364][9] It achieves extremely high peak levels of estradiol but has a very short duration.[60][280][9] Kuhnz et al. (1993) reported that a single intravenous injection of 0.3 mg estradiol resulted in peak estradiol concentrations of 8,321 pg/mL at 5 minutes post-injection.[60] Estradiol levels decreased to 1,628 pg/mL after 30 minutes, to 778 pg/mL after 1 hour, and to 23 pg/mL after 6 hours.[60] Leyendecker et al. (1975) reported that a single intravenous injection of 20 mg estradiol resulted in estradiol levels of 2,950 pg/mL at 12 hours after the injection (earlier time points were not measured).[280] Following this, estradiol levels decreased to around 400 pg/mL by 24 hours post-injection and reached near-baseline levels of 45 pg/mL after 48 hours.[280] The ratio of estradiol to estrone is very high initially (e.g., around 10:1 at peak) but becomes smaller as estradiol levels decline.[60][280] The distribution half-life of intravenous estradiol is about 6 minutes and the terminal half-life of intravenous estradiol is about 0.5 to 2 hours.[10][12][4][9] The peak estradiol levels are far higher and the duration far shorter when estradiol is given by intravenous injection than when estradiol esters are administered by intramuscular or subcutaneous injection.[280][10]
The administration of estradiol valerate by intravenous injection has been studied as well.[4][365] It has been found to be very rapidly cleaved into estradiol in the blood.[4][365] The metabolism of estradiol valerate does not differ with intravenous versus intramuscular injection.[365]
While estradiol itself has not been used clinically by intravenous injection, certain estrogen preparations such as conjugated estrogens and estramustine phosphate are available in formulations indicated for intravenous injection.[366] Both of these medications act in part as prodrugs of estradiol.[367][368][369] The intravenous formulation of conjugated estrogens is available at a dose of 25 mg per injection and is used in the treatment of abnormal uterine bleeding due to its ability to rapidly and temporarily enhance coagulation.[366] It has also been used off-label to treat severe bleeding after hysteroscopic metroplasty and as an emergency contraceptive.[366][370][368] The formulation is given in a single injection but can be repeated after 6 to 12 hours if necessary.[366][370][368] Intravenous estramustine phosphate has a relatively long duration and, like oral estramustine phosphate, is used in the treatment of prostate cancer.[369][371] Estramustine phosphate was initially introduced as an intravenous formulation and was only later introduced as an oral medication.[371] Following introduction of the more convenient oral formulation, intravenous estramustine phosphate has largely been abandoned.[371]
The administration of large doses of estrogens intravenously has been studied.[372][373][374]
-
Baseline-corrected levels of estradiol, estrone, and estrone conjugates (e.g., estrone sulfate) after a single intravenous infusion of 0.3 mg estradiol in aqueous solution in women.[60]
-
Estradiol levels after a short intravenous infusion of 20 mg estradiol in aqueous solution or an intramuscular injection of an equimolar dose of estradiol benzoate, estradiol valerate, or estradiol undecylate in oil solution in women.[281][280] Earlier time points than 12 hours post-infusion for intravenous estradiol were not measured.[281][280]
General
[edit]Absorption
[edit]Estradiol is well-absorbed regardless of route of administration.[10] However, the bioavailability of estradiol differs substantially with different routes of administration.[10][4] Oral estradiol has an average bioavailability of around 5%, requiring relatively high dosages of estradiol for effects.[10] Estradiol administered in the form of an ester by intramuscular or subcutaneous injection has complete bioavailability.[4][332][8]
Distribution
[edit]Estradiol is rapidly distributed throughout the body, with a distribution phase of about 6 minutes following intravenous injection.[12] Estradiol is taken up into cells via passive diffusion due to its lipophilicity.[375] Due to binding to the ERs, estradiol is preferentially concentrated in tissues with the highest ER content.[12] In animals, these tissues have included the uterus, vagina, mammary glands, pituitary gland, hypothalamus, other brain regions, adipose tissue, liver, and adrenal glands, among other tissues.[12][376] In contrast to estradiol, due to its low affinities for the ERs, estrone is not accumulated in target tissues.[10] Estradiol has been found to cross the blood–brain barrier in rhesus monkeys.[12] The volume of distribution of estradiol has been found to be 0.85 to 1.17 L/kg.[12] In another study however, its volume of distribution was only 0.082 ± 0.015 L/kg (4.8 L in women of average weight 58.4 kg).[9]
In terms of plasma protein binding, estradiol is bound loosely to albumin and tightly to SHBG, with approximately 97 to 98% of estradiol bound to plasma proteins.[2] In the circulation, approximately 38% of estradiol is bound to SHBG and 60% is bound to albumin, with 2 to 3% free or unbound.[3] However, with oral estradiol, there is an increase in hepatic SHBG production and hence SHBG levels (e.g., +50%), and this results in a relatively reduced fraction of free estradiol.[1][17] As only free estradiol that is not bound to plasma proteins or SHBG is biologically active, this may reduce the potency of oral estradiol by some degree.[12][17] However, a study found that the free fraction of estradiol was similar with doses of oral and topical estradiol that resulted in equivalent total estradiol levels.[129]
Metabolism
[edit] Metabolic pathways of estradiol in humans
|
There are several major pathways of estradiol metabolism, which occur both in the liver and in other tissues:[12][10][1]
- Dehydrogenation by 17β-hydroxysteroid dehydrogenase (17β-HSD) into estrone
- Conjugation by estrogen sulfotransferases and UDP-glucuronyltransferases into C3 and/or C17β estrogen conjugates like estrone sulfate and estradiol glucuronide
- Hydroxylation by cytochrome P450 enzymes such as CYP1A1 and CYP3A4 into catechol estrogens like 2-hydroxyestrone and 2-hydroxyestradiol as well as 16-hydroxylated estrogens like 16α-hydroxyestrone and estriol (16α-hydroxyestradiol)
The liver is almost entirely responsible for metabolism of estradiol.[377]
Both dehydrogenation of estradiol by 17β-HSD into estrone and conjugation into estrogen conjugates are reversible transformations.[12][10] However, in regards to sulfation and desulfation, transformation of estrone into estrone sulfate is predominant relative to the reverse reaction.[12][110]
Estradiol can also be reversibly converted into long-lived lipoidal estradiol forms like estradiol palmitate and estradiol stearate as a minor route of metabolism.[11]
The elimination half-life of estradiol administered via intravenous injection has been found to be 2 hours in men and 27 to 50 minutes in women.[4][9][378][379] Other routes of administration of estradiol like oral administration or intramuscular injection have far longer elimination half-lives and durations of action due to (1) the formation of a large circulating reservoir of metabolism-resistant estrogen conjugates that can be reconverted back into estradiol and/or (2) the formation of slowly-releasing depots.[12][10]
The metabolic clearance rates of estradiol, estrone, and estrone sulfate are 580 L/day/m2, 1,050 L/day/m2, and 80 L/day/m2, respectively.[10]
Elimination
[edit]A single dose of oral estradiol valerate is eliminated 54% in urine and 6% in feces.[1] A substantial amount of estradiol is also excreted in bile.[1] The urinary metabolites of estradiol are predominantly present in the form of estrogen conjugates, including glucuronides and, to a lesser extent, sulfates.[1] The main metabolites of estradiol in urine are estrone glucuronide (13–30%), 2-hydroxyestrone (2.6–10.1%), unchanged estradiol (5.2–7.5%), estriol (2.0–5.9%), and 16α-hydroxyestrone (1.0–2.9%).[1] Following an intravenous injection of labeled estradiol in women, almost 90% is excreted in urine and feces within 4 to 5 days.[378][379] Enterohepatic recirculation causes a delay in excretion of estradiol.[378]
See also
[edit]- Pharmacodynamics of estradiol
- Pharmacodynamics of progesterone
- Pharmacokinetics of progesterone
- Pharmacokinetics of testosterone
References
[edit]- ^ a b c d e f g h i j k l m n o p q r Stanczyk FZ, Archer DF, Bhavnani BR (June 2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
- ^ a b c d e f O'Connell MB (1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9 Suppl): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713. S2CID 10159196.
- ^ a b c d e f g h i j k Kuhnz W, Blode H, Zimmermann H (1993). "Pharmacokinetics of Exogenous Natural and Synthetic Estrogens and Antiestrogens". Estrogens and Antiestrogens II. Handbook of Experimental Pharmacology. Vol. 135 / 2. pp. 261–322. doi:10.1007/978-3-642-60107-1_15. ISBN 978-3-642-64261-6. ISSN 0171-2004.
- ^ a b c d e f g h i j k l Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ^ a b Falcone T, Hurd WW (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 978-0-323-03309-1.
- ^ Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW (March 1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17 beta-estradiol". Obstetrics and Gynecology. 89 (3): 340–345. doi:10.1016/S0029-7844(96)00513-3. PMID 9052581. S2CID 71641652.
- ^ Naunton M, Al Hadithy AF, Brouwers JR, Archer DF (2006). "Estradiol gel: review of the pharmacology, pharmacokinetics, efficacy, and safety in menopausal women". Menopause. 13 (3): 517–527. doi:10.1097/01.gme.0000191881.52175.8c. PMID 16735950. S2CID 42748448.
- ^ a b c d e Sierra-Ramírez JA, Lara-Ricalde R, Lujan M, Velázquez-Ramírez N, Godínez-Victoria M, Hernádez-Munguía IA, et al. (December 2011). "Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg)". Contraception. 84 (6): 565–570. doi:10.1016/j.contraception.2011.03.014. PMID 22078184.
- ^ a b c d e f g White CM, Ferraro-Borgida MJ, Fossati AT, McGill CC, Ahlberg AW, Feng YJ, et al. (1998). "The pharmacokinetics of intravenous estradiol--a preliminary study". Pharmacotherapy. 18 (6): 1343–1346. doi:10.1002/j.1875-9114.1998.tb03157.x. PMID 9855336. S2CID 9970669.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce cf cg ch ci cj ck cl cm cn co cp cq cr cs ct cu cv cw cx cy cz da db dc dd de df dg dh di dj dk dl dm dn do dp dq dr ds dt du Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 121, 226, 235–237. ISBN 978-3-642-58616-3.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 163–178, 235–237, 252–253, 261–276, 538–543. ISBN 978-3-642-60107-1.
- ^ a b c Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 (Suppl 2): S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384. S2CID 32650111.
- ^ a b c Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD (May 2003). "Transdermal estradiol therapy for advanced prostate cancer--forward to the past?". J. Urol. 169 (5): 1735–7. doi:10.1097/01.ju.0000061024.75334.40. PMID 12686820.
- ^ a b c d e f g h i Lobo RA (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- ^ a b c d Notelovitz M, van Keep PA (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28 – November 2, 1984. Springer Science & Business Media. pp. 397, 399. ISBN 978-94-009-4145-8.
[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/mL to only 13 pg/mL.
- ^ a b c d e f g h i j k l m n o p q r s Christian C, von Schoultz B (15 March 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 9–16, 60. ISBN 978-1-85070-545-1.
The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/mL.
- ^ Müller EE, MacLeod RM (6 December 2012). Neuroendocrine Perspectives. Springer Science & Business Media. pp. 121–. ISBN 978-1-4612-3554-5.
[...] [premenopausal] mean [estradiol] concentration of 150 pg/mL [...]
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- ^ "ARCHITECT Estradiol assay" (PDF). Abbott Laboratories. November 2009. Archived from the original (PDF) on 27 November 2021. Retrieved 20 December 2018.
- ^ Troisi R, Potischman N, Roberts JM, Harger G, Markovic N, Cole B, Lykins D, Siiteri P, Hoover RN (2003). "Correlation of serum hormone concentrations in maternal and umbilical cord samples". Cancer Epidemiol. Biomarkers Prev. 12 (5): 452–6. PMID 12750241.
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- ^ Lobo RA (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- ^ Falcone T, Hurd WW (14 June 2017). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer. pp. 179–. ISBN 978-3-319-52210-4.
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- ^ Kleemann A, Engel J, Kutscher B, Reichert D (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1167–1174. ISBN 978-3-13-179525-0.
- ^ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 276, 454–455, 566–567. ISBN 978-3-7692-2114-5.
- ^ Krishna UR, Sheriar NK (1996). Menopause. Orient Blackswan. pp. 70–. ISBN 978-81-250-0910-8.
- ^ "NNR: Products Recently Accepted by the A. M. A. Council on Pharmacy and Chemistry". Journal of the American Pharmaceutical Association (Practical Pharmacy ed.). 10 (11): 692–694. 1949. doi:10.1016/S0095-9561(16)31995-8. ISSN 0095-9561.
- ^ "AERODIOL (Oestradiol hemihydrate 150 micrograms/actuation)" (PDF). Servier Laboratories (Aust) Pty Ltd.
- ^ "Estradiol". Drugs.com.
- ^ Sahin FK, Koken G, Cosar E, Arioz DT, Degirmenci B, Albayrak R, Acar M (2008). "Effect of Aerodiol administration on ocular arteries in postmenopausal women". Gynecol. Endocrinol. 24 (4): 173–7. doi:10.1080/09513590701807431. PMID 18382901.
300 μg 17β-estradiol (Aerodiol®; Servier, Chambrayles-Tours, France) was administered via the nasal route by a gynecologist. This product is unavailable after March 31, 2007 because its manufacturing and marketing are being discontinued.
- ^ Plouffe Jr L, Ravnikar VA, Speroff L, Watts NB (6 December 2012). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 271–. ISBN 978-1-4612-4330-4.
- ^ Hospital Formulary and Compendium of Useful Information. University of California Press. 1952. pp. 49–. GGKEY:2UAAZRZ5LN0.
- ^ Leidenberger FA (17 April 2013). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 527–. ISBN 978-3-662-08110-5.
- ^ a b c d Meikle AW (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 380–. ISBN 978-1-59259-700-0.
- ^ a b James VH, Pasqualini JR (22 October 2013). Hormonal Steroids: Proceedings of the Sixth International Congress on Hormonal Steroids. Elsevier Science. pp. 821–. ISBN 978-1-4831-9067-9.
- ^ a b c d e f g h i Englund DE, Johansson ED (1981). "Oral versus vaginal absorption in oestradiol in postmenopausal women. Effects of different particles sizes". Upsala Journal of Medical Sciences. 86 (3): 297–307. doi:10.3109/03009738109179241. PMID 7324289.
- ^ Dosage Form Design Considerations. Elsevier Science. 28 July 2018. pp. 159–. ISBN 978-0-12-814424-4.
- ^ a b c d e Henzl MR (1978). "Natural and Synthetic Female Sex Hormones". In Yen SS, Jaffe RB (eds.). Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. W.B. Saunders Co. pp. 421–468. ISBN 978-0-7216-9625-6.
- ^ Stanczyk FZ (1998). "Pharmacological background of estrogen replacement therapy and continuance". In Fraser IS, Jansen RP, Lobo RA, Whitehead MI (eds.). Estrogens and Progestogens in Clinical Practice. Churchill Livingstone. pp. 655–666. ISBN 978-0-443-04706-0.
- ^ a b c Yen SS, Martin PL, Burnier AM, Czekala NM, Greaney MO, Callantine MR (March 1975). "Circulating estradiol, estrone and gonadotropin levels following the administration of orally active 17beta-estradiol in postmenopausal women". The Journal of Clinical Endocrinology and Metabolism. 40 (3): 518–521. doi:10.1210/jcem-40-3-518. PMID 1117058.
- ^ Hammond CB, Maxson WS (June 1986). "Estrogen replacement therapy". Clin Obstet Gynecol. 29 (2): 407–30. doi:10.1097/00003081-198606000-00022. PMID 3522011. S2CID 31166713.
A micronized form of estradiol in which 80% of the particles present are 20 × 106 M or less results in effective oral, sublingual, or vaginal absorption.61
- ^ a b Devroey P, Pados G (1998). "Preparation of endometrium for egg donation". Hum. Reprod. Update. 4 (6): 856–61. doi:10.1093/humupd/4.6.856. PMID 10098476.
Oestradiol valerate and oestradiol in a micronized form are the most widely used oestrogen per os for steroid substitution therapy. Our regimen, as of most other groups [...] is oestradiol valerate (Progynova; Schering, Berlin, Germany) given in various concentrations throughout the cycle [...]. According to Norfolk's protocol, 2 mg of micronized oestradiol valerate are given on cycle days 1–5. [...] In tablet form, micronized oestradiol valerate is also efficiently absorbed [...]
- ^ a b Martin PL, Burnier AM, Greaney MO (May 1972). "Oral menopausal therapy using 17- micronized estradiol. A preliminary study of effectiveness, tolerance and patient preference". Obstetrics and Gynecology. 39 (5): 771–774. PMID 5023261.
- ^ a b Herr F, Revesz C, Manson AJ, Jewell JB (1970). "Biological Properties of Estrogen Sulfates". Chemical and Biological Aspects of Steroid Conjugation. pp. 368–408. doi:10.1007/978-3-642-49793-3_8 (inactive 1 November 2024). ISBN 978-3-642-49506-9.
{{cite book}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Martinez-Manautou J, Rudel HW (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Greenblatt RB (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253. ISBN 9780397590100.
- ^ a b Martinez-Manautou J, Rudel HW (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Robert Benjamin Greenblatt (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253.
- ^ a b Dorfman RI (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 40, 392. ISBN 978-1-4832-7299-3.
Ferin (1952) also studied duration of action in women with estrogen deficiency by recording the days of freedom from hot flushes. He rates estradiol-3-benzoate, estradiol-3-furoate, estradiol dipropionate, estradiol-17-caprylate, estradiol-3-benzoate-17-caprylate in oil, and finally estradiol-3-benzoate in emulsion or as microcrystals in that order of duration of action. After 10 mg. of each of the above preparations, a woman would typically remain free of symptoms for 10 days. This could, however, be as much as 50 days.
- ^ Horsky J, Presl J (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 313–. ISBN 978-94-009-8195-9.
- ^ Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. p. 34. ISBN 978-0-12-137250-7.
- ^ Gibian H, Kopp R, Kramer M, Neumann F, Richter H (1968). "Effect of particle size on biological activity of norethisterone acetate". Acta Physiologica Latino Americana. 18 (4): 323–326. PMID 5753386.
- ^ He CH, Shi YE, Liao DL, Zhu YH, Xu JQ, Matlin SA, et al. (May 1990). "Comparative cross-over pharmacokinetic study on two types of postcoital contraceptive tablets containing levonorgestrel". Contraception. 41 (5): 557–567. doi:10.1016/0010-7824(90)90064-3. PMID 2112080.
- ^ Göretzlehner G, Lauritzen C, Römer T, Rossmanith W (30 November 2011). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 44–. ISBN 978-3-11-024568-4.
- ^ Ryden AB (1950). "Natural and synthetic oestrogenic substances; their relative effectiveness when administered orally". Acta Endocrinologica. 4 (2): 121–139. doi:10.1530/acta.0.0040121. PMID 15432047.
- ^ Ryden A (1947). "Natural and synthetic estrogenic substances; a comparison of the effect upon the endometrium in castrated women". Acta Pathologica et Microbiologica Scandinavica. 24 (3–4): 213–241. doi:10.1111/j.1699-0463.1947.tb00592.x. PMID 18900891.
- ^ Kottmeier HL (1947). "Ueber blutungen in der menopause: Speziell der klinischen bedeutung eines endometriums mit zeichen hormonaler beeinflussung: Part I". Acta Obstetricia et Gynecologica Scandinavica. 27 (s6): 1–121. doi:10.3109/00016344709154486. ISSN 0001-6349. S2CID 81371648.
There is no doubt that the conversion of the endometrium with injections of both synthetic and native estrogenic hormone preparations succeeds, but the opinion whether native, orally administered preparations can produce a proliferation mucosa changes with different authors. PEDERSEN-BJERGAARD (1939) was able to show that 90% of the folliculin taken up in the blood of the vena portae is inactivated in the liver. Neither KAUFMANN (1933, 1935), RAUSCHER (1939, 1942) nor HERRNBERGER (1941) succeeded in bringing a castration endometrium into proliferation using large doses of orally administered preparations of estrone or estradiol. Other results are reported by NEUSTAEDTER (1939), LAUTERWEIN (1940) and FERIN (1941); they succeeded in converting an atrophic castration endometrium into an unambiguous proliferation mucosa with 120–300 mg oestradiol or with 380 mg oestrone.
- ^ Kvorning I, Christensen MS (1981). "Bioavailability of Four Oestradiol Suspensions with Different Particle-Sizes - In Vivo/In Vitro Correlation". Drug Development and Industrial Pharmacy. 7 (3). Informa UK Limited: 289–303. doi:10.3109/03639048109051946. ISSN 0363-9045.
- ^ a b c d e f g h Fotherby K (August 1996). "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception. 54 (2): 59–69. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
- ^ a b c d e f g h i j k Kuhnz W, Gansau C, Mahler M (September 1993). "Pharmacokinetics of estradiol, free and total estrone, in young women following single intravenous and oral administration of 17β-estradiol". Arzneimittelforschung. 43 (9): 966–73. ISSN 0004-4172. PMID 8240460.
- ^ Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgend Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- ^ Lewin A, Pisov G, Turgeman R, Fatum M, Shufaro Y, Simon A, Laufer N, Revel A, Reubinoff B, Safran A (April 2002). "Simplified artificial endometrial preparation, using oral estradiol and novel vaginal progesterone tablets: a prospective randomized study". Gynecol. Endocrinol. 16 (2): 131–6. doi:10.1080/gye.16.2.131.136. PMID 12012623. S2CID 40295755.
- ^ Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, Dickler MN, Silverman P, Fleming GF, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Siegel BA (August 2009). "Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study". JAMA. 302 (7): 774–80. doi:10.1001/jama.2009.1204. PMC 3460383. PMID 19690310.
- ^ a b c Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–197. doi:10.1016/0378-5122(90)90003-o. PMID 2170822.
- ^ a b Fotherby K (1976). "Pharmacology of Natural and Synthetic Estrogens". In Campbell S (ed.). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. pp. 87–95. doi:10.1007/978-94-011-6165-7_7. ISBN 978-94-011-6165-7.
- ^ a b Fruzzetti F, Bitzer J (January 2010). "Review of clinical experience with estradiol in combined oral contraceptives". Contraception. 81 (1): 8–15. doi:10.1016/j.contraception.2009.08.010. PMID 20004267.
- ^ a b Vree TB, Timmer CJ (August 1998). "Enterohepatic cycling and pharmacokinetics of oestradiol in postmenopausal women". The Journal of Pharmacy and Pharmacology. 50 (8): 857–864. doi:10.1111/j.2042-7158.1998.tb04000.x. PMID 9751449. S2CID 23550553.
- ^ a b Timmer CJ, Geurts TB (1999). "Bioequivalence assessment of three different estradiol formulations in postmenopausal women in an open, randomized, single-dose, 3-way cross-over study". European Journal of Drug Metabolism and Pharmacokinetics. 24 (1): 47–53. doi:10.1007/BF03190010. PMID 10412891. S2CID 20513936.
- ^ a b Wiegratz I, Fink T, Rohr UD, Lang E, Leukel P, Kuhl H (September 2001). "[Cross-over comparison of the pharmacokinetics of estradiol during hormone replacement therapy with estradiol valerate or micronized estradiol]" [Cross-over comparison of the pharmacokinetics of estradiol during hormone replacement therapy with estradiol valerate or micronized estradiol]. Zentralblatt für Gynäkologie (in German). 123 (9): 505–512. doi:10.1055/s-2001-18223. PMID 11709743. S2CID 260353858.
- ^ a b c d e f g Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ Lauritzen C (June 1977). "[Estrogen thearpy in practice. 3. Estrogen preparations and combination preparations]" [Estrogen therapy in practice. 3. Estrogen preparations and combination preparations]. Fortschritte Der Medizin (in German). 95 (21): 1388–92. PMID 559617.
- ^ a b c d e f g h i j k Wolf AS, Schneider HP (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 78–. ISBN 978-3-642-75101-1.
- ^ Göretzlehner G, Lauritzen C, Römer T, Rossmanith W (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 44–. ISBN 978-3-11-024568-4.
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- ^ a b c d e Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl J (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598–599. ISBN 978-3-11-150424-7.
- ^ Lauritzen CH (January 1976). "The female climacteric syndrome: significance, problems, treatment". Acta Obstetricia Et Gynecologica Scandinavica. Supplement. 51: 47–61. doi:10.3109/00016347509156433. PMID 779393.
- ^ Lauritzen C (1975). "The Female Climacteric Syndrome: Significance, Problems, Treatment". Acta Obstetricia et Gynecologica Scandinavica. 54 (s51): 48–61. doi:10.3109/00016347509156433. ISSN 0001-6349.
- ^ Kopera H (1991). "Hormone der Gonaden". Hormonelle Therapie für die Frau. Kliniktaschenbücher. pp. 59–124. doi:10.1007/978-3-642-95670-6_6. ISBN 978-3-540-54554-5. ISSN 0172-777X.
- ^ Scott WW, Menon M, Walsh PC (April 1980). "Hormonal Therapy of Prostatic Cancer". Cancer. 45 (Suppl 7): 1929–1936. doi:10.1002/cncr.1980.45.s7.1929. PMID 29603164.
- ^ Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgender Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- ^ Ryden AB (1950). "Natural and synthetic oestrogenic substances; their relative effectiveness when administered orally". Acta Endocrinologica. 4 (2): 121–39. doi:10.1530/acta.0.0040121. PMID 15432047.
- ^ Ryden AB (1951). "The effectiveness of natural and synthetic oestrogenic substances in women". Acta Endocrinologica. 8 (2): 175–91. doi:10.1530/acta.0.0080175. PMID 14902290.
- ^ Kottmeier HL (1947). "Ueber blutungen in der menopause: Speziell der klinischen bedeutung eines endometriums mit zeichen hormonaler beeinflussung: Part I". Acta Obstetricia et Gynecologica Scandinavica. 27 (s6): 1–121. doi:10.3109/00016344709154486. ISSN 0001-6349.
There is no doubt that the conversion of the endometrium with injections of both synthetic and native estrogenic hormone preparations succeeds, but the opinion whether native, orally administered preparations can produce a proliferation mucosa changes with different authors. PEDERSEN-BJERGAARD (1939) was able to show that 90% of the folliculin taken up in the blood of the vena portae is inactivated in the liver. Neither KAUFMANN (1933, 1935), RAUSCHER (1939, 1942) nor HERRNBERGER (1941) succeeded in bringing a castration endometrium into proliferation using large doses of orally administered preparations of estrone or estradiol. Other results are reported by NEUSTAEDTER (1939), LAUTERWEIN (1940) and FERIN (1941); they succeeded in converting an atrophic castration endometrium into an unambiguous proliferation mucosa with 120–300 oestradiol or with 380 oestrone.
- ^ Rietbrock N, Staib AH, Loew D (11 March 2013). Klinische Pharmakologie: Arzneitherapie. Springer-Verlag. pp. 426–. ISBN 978-3-642-57636-2.
- ^ Herr F, Revesz C, Manson AJ, Jewell JB (1970). "Biological Properties of Estrogen Sulfates". Chemical and Biological Aspects of Steroid Conjugation. pp. 368–408. doi:10.1007/978-3-642-49793-3_8. ISBN 978-3-642-49506-9.
- ^ Duncan CJ, Kistner RW, Mansell H (October 1956). "Suppression of ovulation by trip-anisyl chloroethylene (TACE)". Obstetrics and Gynecology. 8 (4): 399–407. PMID 13370006.
- ^ a b c d e f Selby P, McGarrigle HH, Peacock M (March 1989). "Comparison of the effects of oral and transdermal oestradiol administration on oestrogen metabolism, protein synthesis, gonadotrophin release, bone turnover and climacteric symptoms in postmenopausal women". Clinical Endocrinology. 30 (3): 241–249. doi:10.1111/j.1365-2265.1989.tb02232.x. PMID 2512035. S2CID 26077537.
- ^ a b Wang-Cheng R, Neuner JM, Barnabei VM (2007). Menopause. ACP Press. pp. 91–. ISBN 978-1-930513-83-9.
- ^ a b Anderson JJ, Garner SC (24 October 1995). Calcium and Phosphorus in Health and Disease. CRC Press. pp. 215–216. ISBN 978-0-8493-7845-4.
- ^ a b c d e Slater CC, Hodis HN, Mack WJ, Shoupe D, Paulson RJ, Stanczyk FZ (2001). "Markedly elevated levels of estrone sulfate after long-term oral, but not transdermal, administration of estradiol in postmenopausal women". Menopause. 8 (3): 200–203. doi:10.1097/00042192-200105000-00009. PMID 11355042. S2CID 19233142.
- ^ Hogervorst E (24 September 2009). Hormones, Cognition and Dementia: State of the Art and Emergent Therapeutic Strategies. Cambridge University Press. pp. 82–. ISBN 978-0-521-89937-6.
- ^ Wright JV (December 2005). "Bio-identical steroid hormone replacement: selected observations from 23 years of clinical and laboratory practice". Annals of the New York Academy of Sciences. 1057 (1): 506–524. Bibcode:2005NYASA1057..506W. doi:10.1196/annals.1356.039. PMID 16399916. S2CID 38877163.
- ^ a b Friel PN, Hinchcliffe C, Wright JV (March 2005). "Hormone replacement with estradiol: conventional oral doses result in excessive exposure to estrone". Alternative Medicine Review. 10 (1): 36–41. PMID 15771561.
- ^ a b Lobo RA (March 1987). "Absorption and metabolic effects of different types of estrogens and progestogens". Obstetrics and Gynecology Clinics of North America. 14 (1): 143–167. doi:10.1016/S0889-8545(21)00577-5. PMID 3306517.
- ^ a b c d e f g h i j k l m Potts RO, Lobo RA (May 2005). "Transdermal drug delivery: clinical considerations for the obstetrician-gynecologist". Obstetrics and Gynecology. 105 (5 Pt 1): 953–961. doi:10.1097/01.AOG.0000161958.70059.db. PMID 15863530. S2CID 23411589.
- ^ a b Borgelt LM (2010). Women's Health Across the Lifespan: A Pharmacotherapeutic Approach. ASHP. pp. 257–. ISBN 978-1-58528-194-7.
- ^ Russo J, Russo IH (28 June 2011). Molecular Basis of Breast Cancer: Prevention and Treatment. Springer Science & Business Media. pp. 92–. ISBN 978-3-642-18736-0.
- ^ Huether SE, McCance KL (27 December 2013). Understanding Pathophysiology. Elsevier Health Sciences. pp. 845–. ISBN 978-0-323-29343-3.
- ^ Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". Journal of Midwifery & Women's Health. 47 (3): 130–138. doi:10.1016/s1526-9523(02)00233-7. PMID 12071379.
- ^ Escande A, Pillon A, Servant N, Cravedi JP, Larrea F, Muhn P, et al. (May 2006). "Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta". Biochemical Pharmacology. 71 (10): 1459–1469. doi:10.1016/j.bcp.2006.02.002. PMID 16554039.
- ^ a b c Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA (August 1985). "Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement". American Journal of Obstetrics and Gynecology. 152 (8): 1099–1106. doi:10.1016/0002-9378(85)90569-1. PMID 2992279.
- ^ a b Fåhraeus L, Larsson-Cohn U (December 1982). "Oestrogens, gonadotrophins and SHBG during oral and cutaneous administration of oestradiol-17 beta to menopausal women". Acta Endocrinologica. 101 (4): 592–596. doi:10.1530/acta.0.1010592. PMID 6818806.
- ^ De Lignieres B, Basdevant A, Thomas G, Thalabard JC, Mercier-Bodard C, Conard J, et al. (March 1986). "Biological effects of estradiol-17 beta in postmenopausal women: oral versus percutaneous administration". The Journal of Clinical Endocrinology and Metabolism. 62 (3): 536–541. doi:10.1210/jcem-62-3-536. PMID 3080464.
- ^ Kloosterboer HJ, Schoonen WG, Verheul HA (11 April 2008). "Proliferation of Breast Cells by Steroid Hormones and Their Metabolites". In Pasqualini JR (ed.). Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 343–366. ISBN 978-1-4200-5873-4.
- ^ Sasson S, Notides AC (July 1983). "Estriol and estrone interaction with the estrogen receptor. II. Estriol and estrone-induced inhibition of the cooperative binding of [3H]estradiol to the estrogen receptor". The Journal of Biological Chemistry. 258 (13): 8118–8122. doi:10.1016/S0021-9258(20)82036-5. PMID 6863280.
- ^ Lundström E, Conner P, Naessén S, Löfgren L, Carlström K, Söderqvist G (2015). "Estrone - a partial estradiol antagonist in the normal breast". Gynecological Endocrinology. 31 (9): 747–749. doi:10.3109/09513590.2015.1062866. PMID 26190536. S2CID 13617050.
- ^ Martel C, Rhéaume E, Takahashi M, Trudel C, Couët J, Luu-The V, Simard J, Labrie F (March 1992). "Distribution of 17 beta-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues". J. Steroid Biochem. Mol. Biol. 41 (3–8): 597–603. doi:10.1016/0960-0760(92)90390-5. PMID 1314080.
- ^ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 121, 226, 235–237. ISBN 978-3-642-58616-3.
- ^ a b Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H (December 2002). "Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues". J. Clin. Endocrinol. Metab. 87 (12): 5760–8. doi:10.1210/jc.2002-020670. PMID 12466383.
- ^ a b Quirk JG, Wendel GD (1983). "Biologic Effects of Natural and Synthetic Estrogens". In Buchsbaum JJ (ed.). The Menopause. Clinical Perspectives in Obstetrics and Gynecology. pp. 55–75. doi:10.1007/978-1-4612-5525-3_5. ISBN 978-1-4612-5525-3. ISSN 0178-0328.
- ^ Borgelt LM (2010). Women's Health Across the Lifespan: A Pharmacotherapeutic Approach. ASHP. pp. 256–. ISBN 978-1-58528-194-7.
- ^ a b Rezvanpour A, Don-Wauchope AC (March 2017). "Clinical implications of estrone sulfate measurement in laboratory medicine". Crit Rev Clin Lab Sci. 54 (2): 73–86. doi:10.1080/10408363.2016.1252310. PMID 27960570. S2CID 1825531.
- ^ Crandall CJ, Guan M, Laughlin GA, Ursin GA, Stanczyk FZ, Ingles SA, Barrett-Connor E, Greendale GA (July 2008). "Increases in serum estrone sulfate level are associated with increased mammographic density during menopausal hormone therapy". Cancer Epidemiol. Biomarkers Prev. 17 (7): 1674–81. doi:10.1158/1055-9965.EPI-07-2779. PMC 2745228. PMID 18628419.
- ^ Pasqualini JR (17 July 2002). Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 195–. ISBN 978-0-203-90924-9.
- ^ James MR, Skaar TC, Lee RY, MacPherson A, Zwiebel JA, Ahluwalia BS, Ampy F, Clarke R (April 2001). "Constitutive expression of the steroid sulfatase gene supports the growth of MCF-7 human breast cancer cells in vitro and in vivo". Endocrinology. 142 (4): 1497–505. doi:10.1210/endo.142.4.8091. PMID 11250930.
- ^ a b Fritz MA, Speroff L (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 753–. ISBN 978-1-4511-4847-3.
- ^ a b Alkjaersig N, Fletcher AP, de Ziegler D, Steingold KA, Meldrum DR, Judd HL (February 1988). "Blood coagulation in postmenopausal women given estrogen treatment: comparison of transdermal and oral administration". The Journal of Laboratory and Clinical Medicine. 111 (2): 224–228. PMID 2448408.
- ^ a b Nugent AG, Leung KC, Sullivan D, Reutens AT, Ho KK (December 2003). "Modulation by progestogens of the effects of oestrogen on hepatic endocrine function in postmenopausal women". Clinical Endocrinology. 59 (6): 690–698. doi:10.1046/j.1365-2265.2003.01907.x. PMID 14974909. S2CID 40208417.
- ^ a b Isotton AL, Wender MC, Casagrande A, Rollin G, Czepielewski MA (February 2012). "Effects of oral and transdermal estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in patients with hypopituitarism during GH treatment: a randomized study". European Journal of Endocrinology. 166 (2): 207–213. doi:10.1530/EJE-11-0560. PMID 22108915.
- ^ a b Jasonni VM, Bulletti C, Naldi S, Ciotti P, Di Cosmo D, Lazzaretto R, Flamigni C (December 1988). "Biological and endocrine aspects of transdermal 17 beta-oestradiol administration in post-menopausal women". Maturitas. 10 (4): 263–270. doi:10.1016/0378-5122(88)90062-x. PMID 3226336.
- ^ a b Weissberger AJ, Ho KK, Lazarus L (February 1991). "Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women". The Journal of Clinical Endocrinology and Metabolism. 72 (2): 374–381. doi:10.1210/jcem-72-2-374. PMID 1991807.
- ^ a b Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E (May 2007). "Effects of the route of oestrogen administration on IGF-1 and IGFBP-3 in healthy postmenopausal women: results from a randomized placebo-controlled study". Clinical Endocrinology. 66 (5): 626–631. doi:10.1111/j.1365-2265.2007.02783.x. PMID 17492948. S2CID 24086563.
- ^ Dansuk R, Unal O, Karageyim Y, Esim E, Turan C (May 2004). "Evaluation of the effect of tibolone and transdermal estradiol on triglyceride level in hypertriglyceridemic and normotriglyceridemic postmenopausal women". Gynecological Endocrinology. 18 (5): 233–239. doi:10.1080/09513590410001715199. PMID 15346658. S2CID 43326076.
- ^ Notelovitz M (March 2006). "Clinical opinion: the biologic and pharmacologic principles of estrogen therapy for symptomatic menopause". MedGenMed. 8 (1): 85. PMC 1682006. PMID 16915215.
- ^ Goodman MP (February 2012). "Are all estrogens created equal? A review of oral vs. transdermal therapy". Journal of Women's Health. 21 (2): 161–169. doi:10.1089/jwh.2011.2839. PMID 22011208.
- ^ Nachtigall LE, Raju U, Banerjee S, Wan L, Levitz M (2000). "Serum estradiol-binding profiles in postmenopausal women undergoing three common estrogen replacement therapies: associations with sex hormone-binding globulin, estradiol, and estrone levels". Menopause. 7 (4): 243–250. doi:10.1097/00042192-200007040-00006. PMID 10914617. S2CID 3076514.
- ^ Santoro N, Worsley R, Miller KK, Parish SJ, Davis SR (March 2016). "Role of Estrogens and Estrogen-Like Compounds in Female Sexual Function and Dysfunction". J Sex Med. 13 (3): 305–16. doi:10.1016/j.jsxm.2015.11.015. PMID 26944462.
- ^ a b c Nilsson B, Holst J, von Schoultz B (October 1984). "Serum levels of unbound 17 beta-oestradiol during oral and percutaneous postmenopausal replacement therapy". Br J Obstet Gynaecol. 91 (10): 1031–6. doi:10.1111/j.1471-0528.1984.tb03683.x. PMID 6541503. S2CID 5733463.
- ^ a b Kicovic PM, Luisi M, Franchi F, Alicicco E (July 1977). "Effects of orally administered oestradiol decanoate on plasma oestradiol, oestrone and gonadotrophin levels, vaginal cytology, cervical mucus and endometrium in ovariectomized women". Clinical Endocrinology. 7 (1): 73–77. doi:10.1111/j.1365-2265.1977.tb02941.x. PMID 880735. S2CID 13639429.
- ^ a b Luisi M, Kicovic PM, Alicicco E, Franchi F (April 1978). "Effects of estradiol decanoate in ovariectomized women". Journal of Endocrinological Investigation. 1 (2): 101–106. doi:10.1007/BF03350355. PMID 755846. S2CID 38187367.
- ^ a b de Visser J, van der Vies J (June 1977). "Oestrogenic activity of oestradiol-decanoate after oral administration to rodents". Acta Endocrinologica. 85 (2): 422–428. doi:10.1530/acta.0.0850422. PMID 577331.
- ^ a b Chaudhury RR (1 January 1981). Pharmacology of Estrogens. Elsevier Science & Technology Books. p. 36. ISBN 978-0-08-026869-9.
- ^ a b Bastianelli C, Farris M, Rosato E, Brosens I, Benagiano G (November 2018). "Pharmacodynamics of combined estrogen-progestin oral contraceptives 3. Inhibition of ovulation". Expert Review of Clinical Pharmacology. 11 (11): 1085–1098. doi:10.1080/17512433.2018.1536544. PMID 30325245. S2CID 53246678.
- ^ a b Dahlgren E, Crona N, Janson PO, Samsioe G (1985). "Oral replacement with estradiol-cyclooctyl acetate: a new estradiol analogue. Effects on serum lipids, proteins, gonadotrophins, estrogens and uterine endometrial morphology". Gynecologic and Obstetric Investigation. 20 (2): 84–90. doi:10.1159/000298978. PMID 3932144.
- ^ a b Schubert W, Cullberg G (1987). "Ovulation inhibition with 17 beta-estradiol cyclo-octyl acetate and desogestrel". Acta Obstetricia et Gynecologica Scandinavica. 66 (6): 543–547. doi:10.3109/00016348709015732. PMID 2962418. S2CID 73200770.
- ^ a b Schubert W, Cullberg G (1988). "Fat-soluble 17 beta-estradiol: a way of reducing dosage in steroid hormonal substitution?". Acta Obstetricia et Gynecologica Scandinavica. 67 (3): 271–275. doi:10.3109/00016348809004218. PMID 2972162. S2CID 39664429.
- ^ Patel JU, Prankerd RJ, Sloan KB (October 1994). "A prodrug approach to increasing the oral potency of a phenolic drug. 1. Synthesis, characterization, and stability of an O-(imidomethyl) derivative of 17 beta-estradiol". Journal of Pharmaceutical Sciences. 83 (10): 1477–1481. doi:10.1002/jps.2600831022. PMID 7884673.
- ^ Patel J, Katovich MJ, Sloan KB, Curry SH, Prankerd RJ (February 1995). "A prodrug approach to increasing the oral potency of a phenolic drug. Part 2. Pharmacodynamics and preliminary bioavailability of an orally administered O-(imidomethyl) derivative of 17 beta-estradiol". Journal of Pharmaceutical Sciences. 84 (2): 174–178. doi:10.1002/jps.2600840210. PMID 7738796.
- ^ a b Elger W, Wyrwa R, Ahmed G, Meece F, Nair HB, Santhamma B, et al. (January 2017). "Estradiol prodrugs (EP) for efficient oral estrogen treatment and abolished effects on estrogen modulated liver functions". The Journal of Steroid Biochemistry and Molecular Biology. 165 (Pt B): 305–311. doi:10.1016/j.jsbmb.2016.07.008. PMID 27449818. S2CID 26650319.
- ^ a b Ahmed G, Elger W, Meece F, Nair HB, Schneider B, Wyrwa R, Nickisch K (October 2017). "A prodrug design for improved oral absorption and reduced hepatic interaction". Bioorganic & Medicinal Chemistry. 25 (20): 5569–5575. doi:10.1016/j.bmc.2017.08.027. PMID 28886996.
- ^ a b c d e Wren BG, Day RO, McLachlan AJ, Williams KM (June 2003). "Pharmacokinetics of estradiol, progesterone, testosterone and dehydroepiandrosterone after transbuccal administration to postmenopausal women". Climacteric. 6 (2): 104–111. doi:10.1080/cmt.6.2.104.111. PMID 12841880. S2CID 26455195.
- ^ Gass MS, Rebar RW, Cuffie-Jackson C, Cedars MI, Lobo RA, Shoupe D, et al. (October 2004). "A short study in the treatment of hot flashes with buccal administration of 17-beta estradiol". Maturitas. 49 (2): 140–147. doi:10.1016/j.maturitas.2003.12.004. PMID 15474758.
- ^ Chandrasekhara DS, Ali V, Prost HM, Nader-Estekhari S (2002). "Buccal estrogen in toothpaste study: systemic absorption of estradiol in postmenopausal or surgically menopausal women when administered as a component in toothpaste". Fertility and Sterility. 78: S98. doi:10.1016/S0015-0282(02)03639-7. ISSN 0015-0282.
- ^ Perloff WH (January 1950). "Estradiol buccal tablets in the treatment of the menopause". American Journal of Obstetrics and Gynecology. 59 (1): 223–225. doi:10.1016/0002-9378(50)90390-5. PMID 15408716.
- ^ Hall GJ (April 1949). "The buccal administration of estradiol". The Journal of Clinical Endocrinology and Metabolism. 9 (4): 382–384. doi:10.1210/jcem-9-4-382. PMID 18120722.
- ^ Bodor N, Buchwald P (2006). "Brain-Targeted Delivery of Estradiol". American Journal of Drug Delivery. 4 (3): 161–175. doi:10.2165/00137696-200604030-00004. ISSN 1175-9038. S2CID 68203212.
- ^ van der Bijl P, van Eyk AD, Thompson IO (April 1998). "Permeation of 17beta-estradiol through human vaginal and buccal mucosa". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 85 (4): 393–398. doi:10.1016/S1079-2104(98)90063-4. PMID 9574947.
- ^ Nicolazzo JA, Reed BL, Finnin BC (February 2004). "Assessment of the effects of sodium dodecyl sulfate on the buccal permeability of caffeine and estradiol". Journal of Pharmaceutical Sciences. 93 (2): 431–440. doi:10.1002/jps.10559. PMID 14705199.
- ^ Kitano M, Maitani Y, Takayama K, Nagai T (1998). "Buccal absorption through golden hamster cheek pouch in vitro and in vivo of 17β-estradiol from hydrogels containing three types of absorption enhancers". International Journal of Pharmaceutics. 174 (1–2): 19–28. doi:10.1016/S0378-5173(98)00234-8. ISSN 0378-5173.
- ^ Nicolazzo JA, Reed BL, Finnin BC (April 2005). "Enhanced buccal mucosal retention and reduced buccal permeability of estradiol in the presence of padimate O and Azone: a mechanistic study". Journal of Pharmaceutical Sciences. 94 (4): 873–882. doi:10.1002/jps.20240. PMID 15736191.
- ^ a b c d e f g h i j k l m n o p q Pines A, Averbuch M, Fisman EZ, Rosano GM (September 1999). "The acute effects of sublingual 17beta-estradiol on the cardiovascular system". Maturitas. 33 (1): 81–5. doi:10.1016/S0378-5122(99)00036-5. PMID 10585176.
- ^ a b Casper RF, Yen SS (1981). "Rapid absorption of micronized estradiol-17 beta following sublingual administration". Obstet Gynecol. 57 (1): 62–4. PMID 7454177.
- ^ General Practitioner. American Academy of General Practice. April 1954. pp. 168–170.
Diogynets* [...] * brand of estradiol transmucosal tablets, scored: 0.125 mg., 0.25 mg. and 1.0 mg., bottles of 50 and 100.
- ^ Welsh AL (1951). Dermatological Formulary: A Guide for Medical Students and Resident Physicians in Dermatology. Educational Publishers. p. 155.
- ^ University of California (1868–1952) (1952). Hospital Formulary and Compendium of Useful Information. University of California Press. pp. 49–. GGKEY:2UAAZRZ5LN0.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ Spence AW (1953). Clinical Endocrinology. Cassell. p. 547.
- ^ Novak E, Brewer JI, Jones GS, Novak ER (1961). Textbook of gynecology. Williams & Wilkins. p. 120.
- ^ Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW (March 1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17 beta-estradiol". Obstet Gynecol. 89 (3): 340–5. doi:10.1016/S0029-7844(96)00513-3. PMID 9052581. S2CID 71641652.
- ^ Pines A, Fisman EZ, Drory Y, Shapira I, Averbuch M, Eckstein N, Motro M, Levo Y, Ayalon D (1998). "The effects of sublingual estradiol on left ventricular function at rest and exercise in postmenopausal women: an echocardiographic assessment". Menopause. 5 (2): 79–85. doi:10.1097/00042192-199805020-00004. PMID 9689200. S2CID 33257234.
- ^ Kuhl H (2000). "Pharmacology of estradiol and estriol". Menopause Review. 5. Société Européenne de Ménopause: 23–44. ISSN 1272-9868. OCLC 473540298.
- ^ Fisman EZ, Tenenbaum A, Shapira I, Motro M, Pines A (October 1999). "The acute effects of sublingual estradiol on left ventricular diastolic function in normotensive and hypertensive postmenopausal women". Maturitas. 33 (2): 145–52. doi:10.1016/S0378-5122(99)00051-1. PMID 10597879.
- ^ a b Ahokas A, Kaukoranta J, Wahlbeck K, Aito M (May 2001). "Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study". J Clin Psychiatry. 62 (5): 332–6. doi:10.4088/JCP.v62n0504. PMID 11411813.
- ^ a b Ahokas A, Kaukoranta J, Aito M (September 1999). "Effect of oestradiol on postpartum depression". Psychopharmacology. 146 (1): 108–10. doi:10.1007/s002130051095. PMID 10485972. S2CID 23729117.
- ^ a b Ng RC, Hirata CK, Yeung W, Haller E, Finley PR (September 2010). "Pharmacologic treatment for postpartum depression: a systematic review". Pharmacotherapy. 30 (9): 928–41. doi:10.1592/phco.30.9.928. PMID 20795848. S2CID 23053672.
- ^ di Scalea TL, Wisner KL (November 2009). "Pharmacotherapy of postpartum depression". Expert Opin Pharmacother. 10 (16): 2593–607. doi:10.1517/14656560903277202. PMC 2929691. PMID 19874247.
- ^ a b c d e Serhal PF, Craft IL (May 1989). "Oocyte donation in 61 patients". Lancet. 1 (8648): 1185–7. doi:10.1016/S0140-6736(89)92762-1. PMID 2566746. S2CID 21953983.
- ^ a b c d e Serhal P (July 1990). "Oocyte donation and surrogacy". Br. Med. Bull. 46 (3): 796–812. doi:10.1093/oxfordjournals.bmb.a072432. PMID 2207608.
- ^ Lim HH, Jang YH, Choi GY, Lee JJ, Lee ES (January 2019). "Gender affirmative care of transgender people: a single center's experience in Korea". Obstet Gynecol Sci. 62 (1): 46–55. doi:10.5468/ogs.2019.62.1.46. PMC 6333764. PMID 30671393.
When we prescribed estradiol, we preferred sublingual estradiol valerate instead of the oral form for feminizing HT since prior researchers have reported the effectiveness of sublingual administration in maintaining high blood estradiol concentration and low E1/E2 ratio [13].
- ^ Pitha J, Harman SM, Michel ME (February 1986). "Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones". J Pharm Sci. 75 (2): 165–7. doi:10.1002/jps.2600750213. PMID 3958926.
- ^ Hoon TJ, Dawood MY, Khan-Dawood FS, Ramos J, Batenhorst RL (November 1993). "Bioequivalence of a 17 beta-estradiol hydroxypropyl-beta-cyclodextrin complex in postmenopausal women". J Clin Pharmacol. 33 (11): 1116–21. doi:10.1002/j.1552-4604.1993.tb01949.x. PMID 8300895. S2CID 72588405.
- ^ Fridriksdóttir H, Loftsson T, Gudmundsson JA, Bjarnason GJ, Kjeld M, Thorsteinsson T (January 1996). "Design and in vivo testing of 17 beta-estradiol-HP beta CD sublingual tablets". Pharmazie. 51 (1): 39–42. PMID 8999433.
- ^ Brewster ME, Howes J, Griffith W, Garty N, Bodor N, Anderson WR, Pop E (1996). "Intravenous and Buccal 2-Hydroxypropyl-β-Cyclodextrin Formulations of E2-CDS — Phase I Clinical Trials". Proceedings of the Eighth International Symposium on Cyclodextrins. pp. 507–510. doi:10.1007/978-94-011-5448-2_112. ISBN 978-0-7923-4029-4.
- ^ Loftsson T, Gudmundsson JA, Arnadóttir RO, Fridriksdóttir H (May 2003). "Sublingual delivery of 17beta-estradiol from cyclodextrin containing tablets". Pharmazie. 58 (5): 358–9. PMID 12779059.
- ^ Burnier AM, Martin PL, Yen SS, Brooks P (May 1981). "Sublingual absorption of micronized 17beta-estradiol". Am. J. Obstet. Gynecol. 140 (2): 146–50. doi:10.1016/0002-9378(81)90101-0. PMID 6786097.
- ^ Fiet J, Hermano M, Witte J, Villette JM, Haimart M, Gourmel B, Tabuteau F, Rouffy J, Dreux C (September 1982). "Post-menopausal concentrations of plasma oestradiol, oestrone, FSH and LH and of total urinary oestradiol and oestrone after a single sublingual dose of oestradiol-17 beta". Acta Endocrinol. 101 (1): 93–7. doi:10.1530/acta.0.1010093. PMID 6812348.
- ^ Devissaguet JP, Brion N, Lhote O, Deloffre P (1999). "Pulsed estrogen therapy: pharmacokinetics of intranasal 17-beta-estradiol (S21400) in postmenopausal women and comparison with oral and transdermal formulations". Eur J Drug Metab Pharmacokinet. 24 (3): 265–71. doi:10.1007/BF03190030. PMID 10716066. S2CID 22359030.
- ^ Dooley M, Spencer CM, Ormrod D (2001). "Estradiol-intranasal: a review of its use in the management of menopause". Drugs. 61 (15): 2243–2262. doi:10.2165/00003495-200161150-00012. PMID 11772138. S2CID 209145169.
- ^ Lopes P, Rozenberg S, Graaf J, Fernandez-Villoria E, Marianowski L (June 2001). "Aerodiol versus the transdermal route: perspectives for patient preference". Maturitas. 38 (Suppl 1): S31–S39. doi:10.1016/S0378-5122(01)00202-X. PMID 11390122.
- ^ Aronson JK (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 173–. ISBN 978-0-08-093292-7.
- ^ Ryden J, Blumenthal PD (2002). Practical Gynecology: A Guide for the Primary Care Physician. ACP Press. pp. 436–. ISBN 978-0-943126-94-4.
- ^ Sahin FK, Koken G, Cosar E, Arioz DT, Degirmenci B, Albayrak R, Acar M (April 2008). "Effect of Aerodiol administration on ocular arteries in postmenopausal women". Gynecological Endocrinology. 24 (4): 173–177. doi:10.1080/09513590701807431. PMID 18382901. S2CID 205632378.
300 μg 17β-estradiol (Aerodiol®; Servier, Chambrayles-Tours, France) was administered via the nasal route by a gynecologist. This product is unavailable after March 31, 2007 because its manufacturing and marketing are being discontinued.
- ^ "Aerodiol nasal spray (discontinued in the UK - December 2006)". Netdoctor. 19 January 2007.
- ^ "AERODIOL Oestradiol hemihydrate" (PDF). Servier Laboratories (Aust) Pty Ltd. July 2007.
- ^ a b Comprehensive Toxicology. Elsevier Science. 1 December 2017. pp. 2–. ISBN 978-0-08-100612-2.
- ^ a b c d e f g h i Rippe JM (15 March 2013). Lifestyle Medicine, Second Edition. CRC Press. pp. 279–. ISBN 978-1-4398-4542-4.
- ^ a b Buster JE (June 2010). "Transdermal menopausal hormone therapy: delivery through skin changes the rules". Expert Opin Pharmacother. 11 (9): 1489–99. doi:10.1517/14656561003774098. PMID 20426703. S2CID 24104591.
- ^ a b c Shah VP, Maibach HI (29 June 2013). Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer Science & Business Media. pp. 47–50. ISBN 978-1-4899-1262-6.
- ^ a b c Sitruk-Ware R (February 1993). "Percutaneous and transdermal oestrogen replacement therapy". Annals of Medicine. 25 (1): 77–82. doi:10.3109/07853899309147862. PMID 8435193.
- ^ Sitruk-Ware R (January 1989). "Transdermal delivery of steroids". Contraception. 39 (1): 1–20. doi:10.1016/0010-7824(89)90012-7. PMID 2642780.
- ^ Wiedersberg S, Guy RH (September 2014). "Transdermal drug delivery: 30+ years of war and still fighting!" (PDF). Journal of Controlled Release. 190: 150–156. doi:10.1016/j.jconrel.2014.05.022. PMID 24852092. S2CID 13842061.
- ^ Oppenheimer E, Greene RR, Burrill MW (1942). "Percutaneous potency of esterified and nonesterified estradiol". Endocrinology. 30 (2): 317–322. doi:10.1210/endo-30-2-317. ISSN 0013-7227.
- ^ Morgan CF (1963). "A comparison of topical and subcutaneous methods of administration of sixteen oestrogens". Journal of Endocrinology. 26 (3): 317–329. doi:10.1677/joe.0.0260317 (inactive 1 November 2024). ISSN 0022-0795.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b c d e f g h i j k "EstroGel® 0.06% (Estradiol Gel) for Topical Use FDA Label" (PDF). Food and Drug Administration. 2017. Retrieved 22 July 2018.
- ^ Schenkel L, Barlier D, Riera M, Barner A (1986). "Transdermal absorption of estradiol 101 from different body sites is comparable". Journal of Controlled Release. 4 (3): 195–201. doi:10.1016/0168-3659(86)90003-9. ISSN 0168-3659.
- ^ a b Balfour JA, Heel RC (October 1990). "Transdermal estradiol. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of menopausal complaints". Drugs. 40 (4): 561–582. doi:10.2165/00003495-199040040-00006. PMID 2083514. S2CID 46969753.
- ^ Smith K, Riche DM, Henyan N (15 April 2010). Clinical Drug Data (11th ed.). McGraw Hill Professional. ISBN 978-0-07-162686-6.
- ^ a b Feldmann RJ, Maibach HI (February 1967). "Regional variation in percutaneous penetration of 14C cortisol in man". The Journal of Investigative Dermatology. 48 (2): 181–183. doi:10.1038/jid.1967.29. PMID 6020682.
- ^ Iyer R, Mok SF, Savkovic S, Turner L, Fraser G, Desai R, et al. (July 2017). "Pharmacokinetics of testosterone cream applied to scrotal skin". Andrology. 5 (4): 725–731. doi:10.1111/andr.12357. PMID 28334510. S2CID 3595479.
- ^ Kühnert B, Byrne M, Simoni M, Köpcke W, Gerss J, Lemmnitz G, Nieschlag E (August 2005). "Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial". European Journal of Endocrinology. 153 (2): 317–326. doi:10.1530/eje.1.01964. PMID 16061839.
- ^ Berth-Jones J (2016). "Principles of Topical Therapy". Rook's Textbook of Dermatology. Wiley. pp. 1–51. doi:10.1002/9781118441213.rtd0018. ISBN 978-1-118-44121-3.
- ^ Benedetti MS, Whomsley R, Poggesi I, Cawello W, Mathy FX, Delporte ML, et al. (2009). "Drug metabolism and pharmacokinetics". Drug Metabolism Reviews. 41 (3): 344–390. doi:10.1080/10837450902891295. PMC 3086155. PMID 19601718.
- ^ Wester RC, Maibach MI (2 January 2002). "Regional Variation in Percutaneous Absorption". In Bronaugh RL, Maibach HI (eds.). Topical Absorption of Dermatological Products. CRC Press. pp. 33–42. doi:10.3109/9780203904015-6. ISBN 978-0-203-90401-5.
- ^ a b c Re I, Asenjo G, Maximino G, Micheletti L (2005). "Tratamiento del Cáncer de Próstata Avanzado con Estrógenos Transdérmicos Escrotales (ETE)" [Transdermal Scrotal Estrogen Patches (TSEP) in the Treatment of Advanced Prostate Cancer]. Revista Argentina de Urología (in Spanish). 70 (4): 231. ISSN 0325-2531. Archived from the original on 27 January 2020. Retrieved 1 December 2019.
- ^ a b c Wisner KL, Sit DK, Moses-Kolko EL, Driscoll KE, Prairie BA, Stika CS, Eng HF, Dills JL, Luther JF, Wisniewski SR (August 2015). "Transdermal Estradiol Treatment for Postpartum Depression: A Pilot, Randomized Trial". J Clin Psychopharmacol. 35 (4): 389–95. doi:10.1097/JCP.0000000000000351. PMC 4485597. PMID 26061609.
Berlex-sponsored pharmacokinetic studies of the Climara® E2 transdermal system demonstrated an approximate 1:1 ratio of [E2 in mcg] delivered to [serum E2 pg/mL concentration] in menopausal women) [11]. Therefore, we anticipated that E2 doses of 50, 100, 150 and 200 mcg/day would produce 50, 100, 150, and 200 pg/mL serum concentrations.
- ^ Mulcahy JJ (16 November 2007). Male Sexual Function: A Guide to Clinical Management. Springer Science & Business Media. pp. 309–. ISBN 978-1-59745-155-0.
- ^ Lobo RA (May 2011). "Risk of venous thromboembolism by route of administration of estrogen". Menopause. 18 (5): 469–470. doi:10.1097/gme.0b013e318211745b. PMID 21407136.
- ^ Renoux C, Dell'aniello S, Garbe E, Suissa S (June 2010). "Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study". BMJ. 340 (jun03 4): c2519. doi:10.1136/bmj.c2519. PMID 20525678. S2CID 1569339.
- ^ Vinogradova Y, Coupland C, Hippisley-Cox J (January 2019). "Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases". BMJ. 364: k4810. doi:10.1136/bmj.k4810. PMC 6326068. PMID 30626577.
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- ^ Henzl MR, Loomba PK (July 2003). "Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice". J Reprod Med. 48 (7): 525–40. PMID 12953327.
- ^ a b c "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- ^ Bruni V, Pampaloni F (2019). "Hormone Replacement Therapy in Premature Ovarian Insufficiency". Menstrual Cycle Related Disorders. ISGE Series. Springer. pp. 111–142. doi:10.1007/978-3-030-14358-9_10. ISBN 978-3-030-14357-2. ISSN 2197-8735. S2CID 198307066.
- ^ a b Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani EN, Dearnaley D, Parmar M, Abel PD (August 2008). "Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer". BJU Int. 102 (4): 442–5. doi:10.1111/j.1464-410X.2008.07583.x. PMC 2564109. PMID 18422771.
- ^ a b Deutsch MB, Bhakri V, Kubicek K (2015). "Effects of cross-sex hormone treatment on transgender women and men". Obstet Gynecol. 125 (3): 605–10. doi:10.1097/AOG.0000000000000692. PMC 4442681. PMID 25730222.
- ^ Kumar C, McIvor RJ, Davies T, Brown N, Papadopoulos A, Wieck A, Checkley SA, Campbell IC, Marks MN (February 2003). "Estrogen administration does not reduce the rate of recurrence of affective psychosis after childbirth". J Clin Psychiatry. 64 (2): 112–8. doi:10.4088/JCP.v64n0202. PMID 12633118.
- ^ Justice AJ, de Wit H (January 2000). "Acute effects of estradiol pretreatment on the response to d-amphetamine in women". Neuroendocrinology. 71 (1): 51–59. doi:10.1159/000054520. PMID 10644899. S2CID 25517508.
- ^ Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- ^ Bland LB, Garzotto M, DeLoughery TG, Ryan CW, Schuff KG, Wersinger EM, Lemmon D, Beer TM (February 2005). "Phase II study of transdermal estradiol in androgen-independent prostate carcinoma". Cancer. 103 (4): 717–23. doi:10.1002/cncr.20857. PMID 15641029. S2CID 770930.
- ^ a b c d "Prostate Adenocarcinoma TransCutaneous Hormones (PATCH)". ClinicalTrials.gov. U.S. National Library of Medicine. Retrieved 21 June 2019.
- ^ a b Gilbert DC, Duong T, Sydes M, Bara A, Clarke N, Abel P, et al. (May 2018). "Transdermal oestradiol as a method of androgen suppression for prostate cancer within the STAMPEDE trial platform". BJU International. 121 (5): 680–683. doi:10.1111/bju.14153. hdl:10044/1/57083. PMID 29388336. S2CID 13738982.
- ^ a b Singla N, Ghandour RA, Raj GV (March 2019). "Investigational luteinizing hormone releasing hormone (LHRH) agonists and other hormonal agents in early stage clinical trials for prostate cancer". Expert Opinion on Investigational Drugs. 28 (3): 249–259. doi:10.1080/13543784.2019.1570130. PMID 30649971. S2CID 58546028.
- ^ "Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE)". ClinicalTrials.gov. U.S. National Library of Medicine. Retrieved 21 June 2019.
- ^ a b c d Dahl M, Feldman JL, Goldberg JM, Jaberi A (2006). "Physical Aspects of Transgender Endocrine Therapy". International Journal of Transgenderism. 9 (3–4): 111–134. doi:10.1300/J485v09n03_06. ISSN 1553-2739. S2CID 146232471.
- ^ Elami-Suzin M, Schenker JG (2017). "Adolescent Pregnancy and Contraception". Frontiers in Gynecological Endocrinology. ISGE Series. Springer. pp. 199–227. doi:10.1007/978-3-319-41433-1_14. ISBN 978-3-319-41431-7. ISSN 2197-8735.
- ^ "Prescribing Information: Vivelle (estradiol transdermal system)" (PDF). U.S. Food and Drug Administration.
- ^ "Prescribing Information: VIVELLE-DOT (estradiol transdermal system)" (PDF). U.S. Food and Drug Administration.
- ^ "Estradiol patch". Daily Med. U.S. National Library of Medicine.
- ^ "Climara (estradiol transdermal system)" (PDF). U.S. Food and Drug Administration.
- ^ "Menostar (estradiol transdermal system)" (PDF). U.S. Food and Drug Administration.
- ^ "ESTRADIOL patch". Daily Med. U.S. National Library of Medicine.
- ^ "Climara Forte". HPRA. Retrieved 22 December 2018.
- ^ a b c Smith RD, Robinson DE, Delignieres B, Albertson BD, Tomai TP, Zinaman MJ, Simon JA (December 1991). "Effects of vehicle supplementation on total estradiol absorption from a transdermal estradiol delivery system". Fertil. Steril. 56 (6): 1029–33. doi:10.1016/S0015-0282(16)54712-8. PMID 1743317.
- ^ Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433. S2CID 6847203.
- ^ Norman G, Dean ME, Langley RE, Hodges ZC, Ritchie G, Parmar MK, et al. (February 2008). "Parenteral oestrogen in the treatment of prostate cancer: a systematic review". British Journal of Cancer. 98 (4): 697–707. doi:10.1038/sj.bjc.6604230. PMC 2259178. PMID 18268497.
- ^ a b Steg A, Benoit G (October 1979). "Percutaneous 17 beta-estradiol in treatment of cancer of prostate". Urology. 14 (4): 373–375. doi:10.1016/0090-4295(79)90083-9. PMID 227153.
- ^ a b Steg A, Benoit G, Limouzin-Lamotte A, Mahoudeau J, Caillens M, Raichvarg D (1979). "Cancer de la prostate: effets métaboliques des bêta-estradiol par voie percutanée" [Cancer of the prostate: metabolic effect of percutaneous beta-estradiol]. La Nouvelle Presse Médicale. 8 (46): 3801–3802. ISSN 0035-3655.
- ^ a b Steg A, Benoit G, Limouzin-Lamotte A, Mahoudeau J, Caillens M, Raichvarg D (November 1980). "[Cancer of the prostate: metabolic effects of percutaneously administered beta-estradiol]" [Cancer of the prostate: metabolic effects of percutaneously administered beta-estradiol]. Revue Médicale de la Suisse Romande (in French). 100 (11): 895–897. PMID 7466061.
- ^ a b Steg A, Benoit G, Maisonneuve P, Tallet F, Nahoul K, Sulatna Y, Raichwarg D, Limousinlamotte MA (1983). "Étude Comparative du Diéthylstilboestrol et du 17 Bêta-oestradiol Per-cutané dans le Traitement du Cancer de la Prostate" [A comparative study of percutaneous 17 beta-estradiol and diethylstilbestrol in the treatment of prostatic cancer]. Ann Urol (Paris) (in French). 17: 197–202. ISSN 0003-4401.
- ^ a b Steg A, Benoit G (1983). "Étude comparative de fortes doses d'oestradiol-17β administrées par voie per-cutanée et de l'orchidectomie bilatérale dans le traitement du cancer de la prostate" [Prostatic carcinoma. Bilateral orchiectomy versus percutaneous administration of large doses of 17β-estradiol. A comparative study.]. Annales d'Urologie. 17 (5): 286–288. ISSN 0003-4401.
- ^ a b Benoit G (1985). "Que Penser du Traitement Hormonal du Cancer de la Prostate" [Thoughts on the Hormonal Treatment of Prostate Cancer]. Gazette Médicale. 92 (5): 33–39. ISSN 0760-758X.
- ^ a b c d e f g Lauritzen C (March 1987). "[New viewpoints in the treatment of postmenopausal complaints]". Archives of Gynecology and Obstetrics (in German). 242 (1–4): 471–479. doi:10.1007/BF01783219. eISSN 1432-0711. PMID 3688962. S2CID 22450751.
- ^ Hindle WH (1998). "Structure-function relationships and metabolism of estrogens and progestogens". In Fraser IS, Jansen RP, Lobo RA, Whitehead MI (eds.). Estrogens and Progestogens in Clinical Practice. Churchill Livingstone. pp. 187–194. ISBN 978-0-443-04706-0.
- ^ Chang KJ, Lee TT, Linares-Cruz G, Fournier S, de Ligniéres B (April 1995). "Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo". Fertil. Steril. 63 (4): 785–91. doi:10.1016/S0015-0282(16)57482-2. PMID 7890063.
- ^ Spicer DV, Ursin G, Pike MC (May 1996). "Progesterone concentrations--physiologic or pharmacologic?". Fertil. Steril. 65 (5): 1077–8. doi:10.1016/s0015-0282(16)58295-8. PMID 8612843.
- ^ Foidart JM, Colin C, Denoo X, Desreux J, Béliard A, Fournier S, de Lignières B (May 1998). "Estradiol and progesterone regulate the proliferation of human breast epithelial cells". Fertil. Steril. 69 (5): 963–9. doi:10.1016/s0015-0282(98)00042-9. PMID 9591509.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598–. ISBN 978-3-11-150424-7.
- ^ Shahrokh Tehraninejad E, Kabodmehri R, Hosein Rashidi B, Jafarabadi M, Keikha F, Masomi M, Hagholahi F (January 2018). "Trans dermal estrogen (oestrogel) for endometrial preparation in freeze embryo transfer cycle: An RCT". Int J Reprod Biomed (Yazd). 16 (1): 51–56. doi:10.29252/ijrm.16.1.51 (inactive 1 November 2024). PMC 5899770. PMID 29675488.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Scott RT, Ross B, Anderson C, Archer DF (May 1991). "Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol". Obstet Gynecol. 77 (5): 758–64. doi:10.1016/0020-7292(92)90761-7. PMID 2014092. S2CID 3155316.
- ^ Järvinen A, Granander M, Nykänen S, Laine T, Geurts P, Viitanen A (November 1997). "Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing". Br J Obstet Gynaecol. 104 (Suppl 16): 14–8. doi:10.1111/j.1471-0528.1997.tb11562.x. PMID 9389778. S2CID 36677042.
- ^ a b Morton TL, Gattermeir DJ, Petersen CA, Day WW, Schumacher RJ (September 2009). "Steady-state pharmacokinetics following application of a novel transdermal estradiol spray in healthy postmenopausal women". J Clin Pharmacol. 49 (9): 1037–46. doi:10.1177/0091270009339187. PMID 19628730. S2CID 23511531.
- ^ a b "ESTRASORB (estradiol topical emulsion) for topical use" (PDF). Exeltis USA, Inc. U.S. Food and Drug Administration. November 2017.
- ^ a b Simon JA (January 2007). "Estradiol topical emulsion for the treatment of moderate-to-severe vasomotor symptoms associated with menopause". Women's Health. 3 (1): 29–37. doi:10.2217/17455057.3.1.29. PMID 19803862. S2CID 10170446.
- ^ Kaufmann M, Costa SB, Scharl A (27 November 2013). Die Gynäkologie. Springer-Verlag. pp. 106–. ISBN 978-3-662-11496-4.
- ^ Hall G, Blombäck M, Landgren BM, Bremme K (2002). "Effects of vaginally administered high estradiol doses on hormonal pharmacokinetics and hemostasis in postmenopausal women". Fertil. Steril. 78 (6): 1172–7. doi:10.1016/s0015-0282(02)04285-1. PMID 12477507.
- ^ Santen RJ, Pinkerton JV, Liu JH, Matsumoto AM, Lobo RA, Davis SR, Simon JA (June 2020). "Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois". Menopause. 27 (6): 614–624. doi:10.1097/GME.0000000000001556. PMID 32379215. S2CID 218534107.
- ^ Schiff I, Tulchinsky D, Ryan KJ (October 1977). "Vaginal absorption of estrone and 17beta-estradiol". Fertil. Steril. 28 (10): 1063–6. doi:10.1016/S0015-0282(16)42855-4. PMID 908445.
- ^ Vaughn TC, Hammond CB (March 1981). "Estrogen replacement therapy". Clin Obstet Gynecol. 24 (1): 253–83. doi:10.1097/00003081-198103000-00023. PMID 6783358.
- ^ Edman CD (1983). "Estrogen Replacement Therapy". In Buchsbaum JJ (ed.). The Menopause. Clinical Perspectives in Obstetrics and Gynecology. Springer. pp. 77–84. doi:10.1007/978-1-4612-5525-3_6. ISBN 978-1-4612-5525-3. ISSN 0178-0328.
- ^ Rigg LA, Milanes B, Villanueva B, Yen SS (December 1977). "Efficacy of intravaginal and intranasal administration of micronized estradiol-17beta". J. Clin. Endocrinol. Metab. 45 (6): 1261–4. doi:10.1210/jcem-45-6-1261. PMID 591620.
- ^ a b c d e f g h i j k Lauritzen C (December 1986). "[Treatment of disorders of the climacteric by vaginal, rectal and transdermal estrogen substitution]" [Treatment of disorders of the climacteric by vaginal, rectal and transdermal estrogen substitution]. Der Gynakologe (in German). 19 (4): 248–253. PMID 3817597.
- ^ a b c d e Göretzlehner G (1989). "[Efficacy of different estrogens as subject to mode of application]" [Efficacy of different estrogens as subject to mode of application]. Zentralblatt für Gynäkologie (in German). 111 (16): 1093–1100. PMID 2683509.
- ^ Nizza M, Giardinelli M (August 1954). "[Activity of natural and synthetic estrogens administered by the rectal route: study by vaginal smears]" [Activity of natural and synthetic estrogens administered by the rectal route: study by vaginal smears]. Minerva Ginecologica (in Italian). 6 (15): 548–551. PMID 13203215.
- ^ Göretzlehner GS (1990). "Wirkspektrum unterschiedlicher Östrogene" [Spectrum of different estrogens]. In Wolf AS, Schneider HP (eds.). Östrogene in Diagnostik und Therapie [Estrogens in diagnostics and therapy] (in German). Springer-Verlag. pp. 77–83. doi:10.1007/978-3-642-75101-1_9. ISBN 978-3-642-75101-1.
- ^ a b c d e f Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548, 551. ISBN 978-3-642-96158-8.
- ^ a b Moran DJ, McGarrigle HH, Lachelin GC (January 1994). "Maternal plasma progesterone levels fall after rectal administration of estriol". The Journal of Clinical Endocrinology & Metabolism. 78 (1): 70–2. doi:10.1210/jcem.78.1.8288717. PMID 8288717.
- ^ Norman AW, Litwack G (23 October 1997). Hormones. Academic Press. pp. 398–. ISBN 978-0-08-053413-8.
- ^ Kurjak A, Chervenak FA (25 September 2006). Textbook of Perinatal Medicine, Second Edition. CRC Press. pp. 699–. ISBN 978-1-4398-1469-7.
- ^ Greene MF, Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore T (25 November 2008). Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice E-Book. Elsevier Health Sciences. pp. 115–. ISBN 978-1-4377-2135-5.
- ^ a b c Emmens CW (22 October 2013). Hormone Assay. Elsevier Science. pp. 394–. ISBN 978-1-4832-7286-3.
- ^ a b c d Ferin J (January 1952). "Relative duration of action of natural and synthetic estrogens administered parenterally in women with estrogen deficiency". The Journal of Clinical Endocrinology & Metabolism. 12 (1): 28–35. doi:10.1210/jcem-12-1-28. PMID 14907837.
- ^ Hochberg RB (June 1998). "Biological esterification of steroids". Endocrine Reviews. 19 (3): 331–48. doi:10.1210/edrv.19.3.0330. PMID 9626557.
- ^ a b c Arun N, Narendra M, Shikha S (15 December 2012). Progress in Obstetrics and Gynecology—3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 416–418. ISBN 978-93-5090-575-3.
- ^ a b c d e f g h Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/S0010-7824(80)80018-7. PMID 7389356.
- ^ Cantor EB (September 1956). "A survey of estrogens". Postgraduate Medicine. 20 (3): 224–231. doi:10.1080/00325481.1956.11691266. PMID 13359169.
- ^ Brown JB (December 1957). "The relationship between urinary oestrogens and oestrogens produced in the body". The Journal of Endocrinology. 16 (2): 202–212. doi:10.1677/joe.0.0160202. PMID 13491750.
- ^ Beer CT, Gallagher TF (May 1955). "Excretion of estrogen metabolites by humans. I. The fate of small doses of estrone and estradiol-17beta". The Journal of Biological Chemistry. 214 (1): 335–349. doi:10.1016/S0021-9258(18)70972-1. PMID 14367392.
- ^ Diczfalusy E, Lauritzen C (1961). Oestrogene beim Menschen: mit 86 Abbildungen. Springer-Verlag. p. 436. ISBN 9783540026433.
- ^ Mazer C, Israel SL, Charny CW (1951). Diagnosis and treatment of menstrual disorders and sterility. P. B. Hoeber. p. 46.
We have, moreover, thus ascertained that, regardless of how large a dose of these estrogens dissolved in oil is administered intramuscularly, no trace of the product is demonstrable in the blood and urine on the fifth day, indicating that the parenteral administration of estrogens in oil should be at intervals no longer than four days (Fig. 17). There is, to the best of our knowledge, only one exception to this rule, namely estradiol dipropionate in oil. This estrogen may be given at weekly intervals because it is more slowly absorbed and eliminated than the other oily preparations of estrogen.
- ^ a b c d e f g h i j k l Leyendecker G, Geppert G, Nocke W, Ufer J (May 1975). "[Estradiol-17beta, estrone, LH and FSH in serum after administration of estradiol-17beta, estradiolbenzoate, estradiol-valeriate and estradiol-undecylate in the female (author's transl)]" [Estradiol 17β, estrone, LH and FSH in serum after administration of estradiol 17β, estradiol benzoate, estradiol valeriate and estradiol undecylate in the female]. Geburtshilfe und Frauenheilkunde (in German). 35 (5): 370–374. PMID 1150068.
- ^ a b c d Geppert G (1975). Untersuchungen zur Pharmakokinetik von Östradiol-17β, Östradiol-Benzoat, Östradiol-Valerianat und Östradiol-Undezylat bei der Frau: der Verlauf der Konzentrationen von Östradiol-17β, Östron, LH und FSH im Serum [Studies on the pharmacokinetics of estradiol-17β, estradiol benzoate, estradiol valerate, and estradiol undecylate in women: the progression of serum estradiol-17β, estrone, LH, and FSH concentrations]. pp. 1–34. OCLC 632312599.
- ^ a b c Lauritzen C (1988). "Natürliche und Synthetische Sexualhormone – Biologische Grundlagen und Behandlungsprinzipien" [Natural and Synthetic Sexual Hormones – Biological Basis and Medical Treatment Principles]. In Schneider HP, Lauritzen C, Nieschlag E (eds.). Grundlagen und Klinik der Menschlichen Fortpflanzung [Foundations and Clinic of Human Reproduction] (in German). Walter de Gruyter. pp. 229–306. ISBN 978-3-11-010968-9. OCLC 35483492.
- ^ a b Ulrich U, Pfeifer T, Lauritzen C (1994). "Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report". Horm. Metab. Res. 26 (9): 428–31. doi:10.1055/s-2007-1001723. PMID 7835827. S2CID 260169203.
- ^ AMA Drug Evaluations. Americal Medical Association. 1971. p. 318.
- ^ Drug Topics Redbook. Topics Publishing Company. 1976. p. 580.
- ^ Krantz JC, Carr CJ, Aviado DM (1972). Krantz and Carr's Pharmacologic principles of medical practice: a textbook on pharmacology and therapeutics for students and practitioners of medicine, pharmacy, and dentistry. Williams & Wilkins. p. 1258. ISBN 9780683002928.
- ^ Howard ME (1949). Modern Drug Encyclopedia and Therapeutic Index. Drug Publications. p. 697.
- ^ Vademecum International. J. Morgan Jones Publications. 1959. p. 147.
- ^ Meyers FH, Jawetz E, Goldfien A (1978). Review of Medical Pharmacology. Lange Medical Pub. p. 400. ISBN 978-0-87041-151-9.
- ^ a b c Osol A, Pratt R (1973). The United States dispensatory. Lippincott. p. 498. ISBN 978-0-397-55901-5.
The following dosages for estradiol in the form of aqueous suspension injected intramuscularly, or pellets implanted subcutaneously, are recommended by a leading maunfacturer: Menopausal syndrome.—In average cases, 1 mg. intramuscularly 2 or 3 times weekly for 2 or 3 weeks; in more severe cases, 1 to 1.5 mg. Thereafter dosage is reduced to the minimum requirement, usually within the range of 0.5 to 1 mg. of estradiol twice weekly.
- ^ Goodman LS (1980). Goodman and Gilman's The Pharmacological Basis of Therapeutics. Macmillan. p. 1428. ISBN 978-0-02-344720-4.
Estradiol, U.S.P. (AQUADiOL, PROGYNON, others), is available in aqueous suspension containing 0.5 or 1 mg/mL for intramuscular injection and as 25-mg pellets for subcutaneous implantation. Various esters of estradiol (benzoate, cypionate, enanthate, propionate, undecylate, and valerate) are prepared in aqueous suspensions or oily solutions for slow release after intramuscular injection. These preparations contain 0.5 to 40 mg/mL and are sold under various trade names (DELESTROGEN, DEPO-ESTRADIOL, OVOCYLIN, many others). Polyestradiol phosphate (ESTRADURIN) is also available for intramuscular use in prostatic carcinoma. Various sulfate esters of Estrone, U.S.P., are available in tablets containing 0.75 to 6 mg (OGEN, others). These esters and estrone are also supplied under various trade names in aqueous suspension and oily solution containing 1 to 5 mg/mL for intramuscular injection.
- ^ a b Newman GT (September 1950). "Estrogen therapy by implantation of estradiol crystals". Am. J. Obstet. Gynecol. 60 (3): 661–4. doi:10.1016/0002-9378(50)90438-8. PMID 14771156.
- ^ "AGOFOLLIN DEPOT Injekční suspenze (Estradioli benzoas)" [AGOFOLLIN DEPOT Suspension for injection (Estradiol benzoase)] (PDF). www.sukl.cz (in Czech). Archived from the original (PDF) on 19 May 2019. Retrieved 15 January 2022.
- ^ "FOLIVIRINE Suspension for injection (Estradiol benzoate, testosterone isobutyrate)" (PDF). Biotika AS (in Czech).
- ^ von Wattenwyl H (1944). "Über eine neue Anwendungsart oestrogener Substanzen" [A new type of application of estrogenic substances]. Schweiz. Med. Wochenschr. (in German). 74: 159–161.
- ^ a b Toppozada HK (1950). "Oestrogenic Therapy with Prolonged Action". Obstetrical & Gynecological Survey. 5 (4): 531. doi:10.1097/00006254-195008000-00021. ISSN 0029-7828.
- ^ a b Field-Richards S (April 1955). "A preliminary series of cases of uterine hypoplasia treated by local injection of an oestrogenic emulsion". The Journal of Obstetrics and Gynaecology of the British Empire. 62 (2): 205–213. doi:10.1111/j.1471-0528.1955.tb14121.x. PMID 14368390. S2CID 41256797.
Oestradiol monobenzoate or oestradiol diproprionate are slowly absorbed from oily solution after intramuscular injection and for this purpose are to be preferred to the unesterified form. As an even slower absorption of oestradiol monobenzoate can be obtained from an aqueous emulsion of this hormone (Lens, Overbeek and Polderman, 1949). Such a preparation for parenteral use was made available for this experiment by Messrs. Organon Laboratories Limited.
- ^ a b c d Lens J, Overbeek GA, Polderman J (1949). "The effect of sex hormones in some organic solvents; emulsified in water". Acta Endocrinologica. 2 (4): 396–404. doi:10.1530/acta.0.0020396. PMID 18140399.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl J (eds.). Ovarian Function and its Disorders. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Kaiser R (September 1961). "[Estrogen excretion during the cycle and after injection of estradiol esters. A contribution to therapy with depot estrogens]" [Estrogen excretion during the cycle and after injection of estradiol esters. A contribution to therapy with depot estrogens]. Geburtshilfe und Frauenheilkunde (in German). 21: 868–878. PMID 13750804.
- ^ Kaiser R (1962). "Über die Oestrogenausscheidung nach Injektion von Oestradiolestern" [Estrogen excretion after injection of estradiol esters]. Gewebs- und Neurohormone [Tissue and Neurohormones: Physiology of the Melanophore Hormone] (in German). Springer, Berlin, Heidelberg. pp. 227–232. doi:10.1007/978-3-642-86860-3_24. ISBN 978-3-540-02909-0.
- ^ Shearman RP (June 1975). "The development of depot contraceptives". Journal of Steroid Biochemistry. 6 (6): 899–902. doi:10.1016/0022-4731(75)90323-4. PMID 1177432.
- ^ a b c Edkins RP (1959). "The Modification of the Duration of Drug Action". Journal of Pharmacy and Pharmacology. 11 (S1): 54T–66T. doi:10.1111/j.2042-7158.1959.tb10412.x. ISSN 0022-3573. S2CID 78850713.
- ^ Herrmann U (1958). "Abhängigkeit der durch Oestrogen- und Progesteron-Kristalle induzierten Abbruchblutung von der Korngröße". Gynecologic and Obstetric Investigation. 146 (4): 318–323. doi:10.1159/000306607. ISSN 1423-002X.
- ^ d'Arcangues C, Snow RC (1999). "Injectable Contraceptives". Fertility Control — Update and Trends. Springer. pp. 121–149. doi:10.1007/978-3-642-86696-8_6. ISBN 978-3-642-86698-2.
Chemists from 12 countries worldwide synthesized 230 ester oximes and esters of norethisterone and levonorgestrel and 72 esters of testosterone. After purification and formulation, these compounds were tested in rodents, and in sub-human primates for the most promising ones. From these biological studies, it emerged that levonorgestrel esters were usually longer acting than the norethisterone esters; aqueous suspensions were generally better than oily solutions; and the duration of action of the longest acting agents was highly dependent on the crystal size of their aqueous suspensions.
- ^ a b c d e Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–385. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
DMPA is a microcrystalline aqueous suspension of medroxyprogesterone acetate which is given by deep intramuscular injection. As a result of the very low solubility in aqueous solution, it provides very prolonged release from the depot site. [...] The exact formulation and the size of the microcrystals is most important for duration of action. The smaller particles are more rapidly dissolved than larger ones and, hence, MPA appears more rapidly in the circulation, with more rapid elimination from the body. This is also true for the once-a-month formulation, Cyclofem.
- ^ a b c Fraser IS (1989). "Systemic hormonal contraception by non-oral routes". In Filshie M, Guillebaud J (eds.). Contraception: Science and Practice. Elsevier Science. pp. 109–125. ISBN 978-1-4831-6366-6.
The more traditional intramuscular injectable methods consist of steroid acetates or esters which have been formulated in oily solutions or microcrystalline suspensions. The steroid esters in oily solution appear to be distributed to storage sites in adipose tissue from which they are slowly released into the circulation. The active steroid moiety is then cleaved from the ester after which it is able to exert its biological effect. Microcrystalline suspensions remain as a depot at the site of injection and the active steroid or ester is slowly released from the surface of the crystals.
- ^ Brown WE, Bradbury JT (August 1949). "The use of the human vaginal smear in the assay of estrogens". The Journal of Clinical Endocrinology and Metabolism. 9 (8): 725–735. doi:10.1210/jcem-9-8-725. PMID 18133489.
- ^ Rappaport F, LASS N (August 1947). "Injectable crystalline hormones". Lancet. 2 (6469): 296–297. doi:10.1016/s0140-6736(47)92066-7. PMID 20344499.
- ^ a b Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 314–322. ISBN 978-0-9828280-1-4.
- ^ a b Polderman J (February 1962). "Suspensions in pharmaceutical practice". Boll Chim Farm. 101 (12): 105–22. doi:10.1093/ajhp/19.12.611. PMID 14487508.
- ^ Gordon D, Horwitt BN, Segaloff A, Murison PJ, Schlosser JV (March 1952). "Hormonal therapy in cancer of the breast. III. Effect of progesterone on clinical course and hormonal excretion". Cancer. 5 (2): 275–7. doi:10.1002/1097-0142(195203)5:2<275::aid-cncr2820050213>3.0.co;2-h. PMID 14905411.
- ^ Bradbury JT, Long RC, Durham WC (1953). "Progesterone and estrogen requirements to induce and maintain decidua". Fertil. Steril. 4 (1): 63–75. doi:10.1016/s0015-0282(16)31145-1. PMID 13021207.
- ^ Fraser IS (1998). Estrogens and Progestogens in Clinical Practice. Churchill Livingstone. p. 13. ISBN 978-0-443-04706-0.
- ^ a b c Kahr H (8 March 2013). Konservative Therapie der Frauenkrankheiten: Anzeigen, Grenzen und Methoden Einschliesslich der Rezeptur. Springer-Verlag. pp. 20–. ISBN 978-3-7091-5694-0.
- ^ Kimmig J (14 March 2013). Therapie der Haut- und Geschlechtskrankheiten. Springer-Verlag. pp. 508–. ISBN 978-3-642-94850-3.
- ^ Overbeek CA (1952). "Some Data on Emulsions of Steroid Hormones". Ciba Foundation Symposium - Steroid Hormone Administration (Book II of Colloquia on Endocrinology, Vol. 3). Novartis Foundation Symposia. John Wiley & Sons. pp. 254–262. doi:10.1002/9780470715154.ch2. ISBN 978-0-470-71515-4. ISSN 1935-4657.
- ^ a b Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. PMID 10230677.
- ^ a b c "All Medicines – Pharmanovia". Archived from the original on 29 March 2019. Retrieved 28 April 2019.
- ^ a b c d Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (May 1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057. S2CID 33548251.
- ^ "Polyestradiol Phosphate". Drugs.com.
- ^ a b c d "Juvenum E (estradiol) – Medicamentos PLM". Medicamentos PLM. Archived from the original on 18 September 2018. Retrieved 17 September 2018.
- ^ "Juvenum E". Drugs.com. Archived from the original on 18 September 2018. Retrieved 20 December 2018.
- ^ a b c Davis ME, Wiener M, Jacobson HI, Jensen EV (December 1963). "Estradiol metabolism in pregnant and nonpregnant women". Am. J. Obstet. Gynecol. 87 (8): 979–90. doi:10.1016/0002-9378(63)90091-7. PMID 14089314.
- ^ a b c Jones TM, Fang VS, Landau RL, Rosenfield R (December 1978). "Direct inhibition of Leydig cell function by estradiol". J. Clin. Endocrinol. Metab. 47 (6): 1368–73. doi:10.1210/jcem-47-6-1368. PMID 122429.
- ^ a b Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ^ a b Schwartz MM, Soule SD (July 1955). "Estradiol 17-beta-cyclopentylpropionate, a longacting estrogen". Am. J. Obstet. Gynecol. 70 (1): 44–50. doi:10.1016/0002-9378(55)90286-6. PMID 14388061.
- ^ a b Gerhard Geppert (1975). Untersuchungen zur Pharmakokinetik von Östradiol-17β, Östradiol-Benzoat, Östradiol-Valerianat und Östradiol-Undezylat bei der Frau: der Verlauf der Konzentrationen von Östradiol-17β, Östron, LH und FSH im Serum [Studies on the pharmacokinetics of estradiol-17β, estradiol benzoate, estradiol valerate, and estradiol undecylate in women: the progression of serum estradiol-17β, estrone, LH, and FSH concentrations]. pp. 1–34. OCLC 632312599.
- ^ a b Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- ^ a b Espino y Sosa, Salvador; Cortés Fuentes, Myriam; Alejandro Gómez Rico, Jacobo; Cortés Bonilla, Manuel (2019). "Non-polymeric Microspheres for the Therapeutic Use of Estrogens: An Innovative Technology". In Khan, Wahid Ali (ed.). Estrogen. ISBN 978-1-83880-867-9.
- ^ "Juvenum E (estradiol) – Medicamentos PLM". Medicamentos PLM. Archived from the original on 18 September 2018. Retrieved 17 September 2018.
- ^ a b Unger CA (December 2016). "Hormone therapy for transgender patients". Translational Andrology and Urology. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
- ^ Al-Futaisi AM, Al-Zakwani IS, Almahrezi AM, Morris D (December 2006). "Subcutaneous administration of testosterone. A pilot study report". Saudi Medical Journal. 27 (12): 1843–1846. PMID 17143361.
- ^ Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M (September 2014). "Subcutaneous Testosterone: An Effective Delivery Mechanism for Masculinizing Young Transgender Men". LGBT Health. 1 (3): 165–167. doi:10.1089/lgbt.2014.0018. PMID 26789709.
- ^ Spratt DI, Stewart II, Savage C, Craig W, Spack NP, Chandler DW, et al. (July 2017). "Subcutaneous Injection of Testosterone Is an Effective and Preferred Alternative to Intramuscular Injection: Demonstration in Female-to-Male Transgender Patients". The Journal of Clinical Endocrinology and Metabolism. 102 (7): 2349–2355. doi:10.1210/jc.2017-00359. PMID 28379417. S2CID 3822101.
- ^ McFarland J, Craig W, Clarke NJ, Spratt DI (August 2017). "Serum Testosterone Concentrations Remain Stable Between Injections in Patients Receiving Subcutaneous Testosterone". Journal of the Endocrine Society. 1 (8): 1095–1103. doi:10.1210/js.2017-00148. PMC 5686655. PMID 29264562.
- ^ Wilson DM, Kiang TK, Ensom MH (March 2018). "Pharmacokinetics, safety, and patient acceptability of subcutaneous versus intramuscular testosterone injection for gender-affirming therapy: A pilot study". American Journal of Health-System Pharmacy. 75 (6): 351–358. doi:10.2146/ajhp170160. PMID 29367424. S2CID 3886536.
- ^ Singh GK, Turner L, Desai R, Jimenez M, Handelsman DJ (July 2014). "Pharmacokinetic-pharmacodynamic study of subcutaneous injection of depot nandrolone decanoate using dried blood spots sampling coupled with ultrapressure liquid chromatography tandem mass spectrometry assays". The Journal of Clinical Endocrinology and Metabolism. 99 (7): 2592–2598. doi:10.1210/jc.2014-1243. PMID 24684468.
- ^ Laurenzano SE, Newfield RS, Lee E, Marinkovic M (December 2021). "Subcutaneous Testosterone Is Effective and Safe as Gender-Affirming Hormone Therapy in Transmasculine and Gender-Diverse Adolescents and Young Adults: A Single Center's 8-Year Experience". Transgender Health. 6 (6): 343–352. doi:10.1089/trgh.2020.0103. PMC 8664110. PMID 34988290. S2CID 234034901.
- ^ a b Chan VO, Colville J, Persaud T, Buckley O, Hamilton S, Torreggiani WC (June 2006). "Intramuscular injections into the buttocks: are they truly intramuscular?". European Journal of Radiology. 58 (3): 480–484. doi:10.1016/j.ejrad.2006.01.008. PMID 16495027.
- ^ a b Palma S, Strohfus P (November 2013). "Are IM injections IM in obese and overweight females? A study in injection technique". Applied Nursing Research. 26 (4): e1–e4. doi:10.1016/j.apnr.2013.09.002. PMID 24156877.
- ^ Kuhl H, Taubert HD (1987). Das Klimakterium – Pathophysiologie, Klinik, Therapie [The Climacteric – Pathophysiology, Clinic, Therapy] (in German). Stuttgart, Germany: Thieme Verlag. p. 108. ISBN 978-3-13-700801-9.
- ^ a b Sherif K (2013). "The Significance of Hormone Routes of Administration". Hormone Therapy. Springer. pp. 57–61. doi:10.1007/978-1-4614-6268-2_7. ISBN 978-1-4614-6267-5.
- ^ a b c d Symonds IM, Arulkumaran S (3 August 2013). Essential Obstetrics and Gynaecology E-Book. Elsevier Health Sciences. pp. 258–. ISBN 978-0-7020-5475-4.
- ^ a b c d e f g h i j k l m n o p q r Plouffe Jr L, Ravnikar VA, Speroff L, Watts NB (6 December 2012). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 271–. ISBN 978-1-4612-4330-4.
- ^ van Boxtel CJ, Santoso B, Edwards IR (2008). Drug Benefits and Risks: International Textbook of Clinical Pharmacology. IOS Press. pp. 769–. ISBN 978-1-58603-880-9.
- ^ a b Birkhauser M, Barlow D, Notelovitz M, Rees M (12 August 2005). Health Plan for the Adult Woman: Management Handbook. CRC Press. pp. 27–. ISBN 978-0-203-49009-9.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. pp. 404–406. ISBN 978-3-88763-075-1. Retrieved 13 September 2012.
- ^ Melville C (22 September 2015). Sexual and Reproductive Health at a Glance. Wiley. pp. 108–. ISBN 978-1-119-23516-3.
- ^ "Estradiol". Drugs.com.
- ^ Pinkerton JV (December 2014). "What are the concerns about custom-compounded "bioidentical" hormone therapy?". Menopause. 21 (12): 1298–300. doi:10.1097/GME.0000000000000376. PMID 25387347.
- ^ Pinkerton JV, Pickar JH (February 2016). "Update on medical and regulatory issues pertaining to compounded and FDA-approved drugs, including hormone therapy". Menopause. 23 (2): 215–23. doi:10.1097/GME.0000000000000523. PMC 4927324. PMID 26418479.
- ^ Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (April 2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". The Journal of Clinical Endocrinology & Metabolism. 101 (4): 1318–43. doi:10.1210/jc.2016-1271. PMID 27032319. S2CID 33802990.
- ^ Khot A, Polmear A (18 November 2011). Practical General Practice E-Book: Guidelines for Effective Clinical Management. Elsevier Health Sciences. pp. 338–. ISBN 978-0-7020-4909-5.
- ^ Kronenberg F (1990). "Hot flashes: epidemiology and physiology". Ann. N. Y. Acad. Sci. 592 (1): 52–86, discussion 123–33. Bibcode:1990NYASA.592...52K. doi:10.1111/j.1749-6632.1990.tb30316.x. PMID 2197954. S2CID 86458927.
- ^ Gearhart JP, Witherington R, Coleman CH (January 1981). "Subcutaneous estradiol pellet implantation in management of advanced prostatic carcinoma". Urology. 17 (1): 44–8. doi:10.1016/0090-4295(81)90010-8. ISSN 0090-4295. PMID 6161467.
- ^ Okie MV, Carden ML, McGee HJ, Tracey EM (March 1951). "Estradiol pellet implantation in carcinoma of the prostate". N Y State J Med. 51 (5): 637–40. ISSN 0028-7628. PMID 14815766.
- ^ Tracey EM (June 1952). "The use of estradiol pellets in the treatment of prostatic carcinoma; reference to variation in response to steroid therapy". J Int Coll Surg. 17 (6): 849–52. ISSN 0096-557X. PMID 14938629.
- ^ Kearns WM (1942). "Treatment of cancer of the prostate with estrogens". Wisconsin Med. J. (41): 575. ISSN 0043-6542.
- ^ Kearns WM (1942). "Pellet Implantation of Hormones in Urology". Journal of Urology. 47 (5): 587–590. doi:10.1016/S0022-5347(17)70847-6. ISSN 0022-5347.
- ^ Ufer J (1968). "Die therapeutische Anwendung der Gestagene beim Menschen" [Therapeutic Use of Progestagens in Humans]. Die Gestagene [Progestogens]. Springer-Verlag. pp. 1026–1124. doi:10.1007/978-3-642-99941-3_7. ISBN 978-3-642-99941-3.
Combination with intrauterine estrogen treatment: In order to achieve a growth effect on the uterus with relatively small doses of estrogens, individual authors [235,264] applied intrauterine or intramural crystal suspensions. In an effort to maintain a regular cycle during this regimen, Husslein and Gitsch [418] injected 30 times 10 mg progesterone parenterally 14 days after topical treatment with 3–10 mg estradiol crystal suspension. In this way, they believe they have achieved maximum uterine development with a minimum of hormones.
- ^ Husslein H, Gitsch E (1951). "Uber die intrauterine Applikation von Ostrogenen in Kristallsuspensionsform" [Intrauterine application of estrogen crystals in suspension]. Zentralbl Gynakol (in German). 73 (14): 1219–24. PMID 14867680.
- ^ Goh HH, Chew PC, Karim SM, Ratnam SS (February 1980). "Control of gonadotrophin secretion by steroid hormones in castrated male transsexuals. I. Effects of oestradiol infusion on plasma levels of follicle-stimulating hormone and luteinizing hormone". Clin. Endocrinol. (Oxf). 12 (2): 165–75. doi:10.1111/j.1365-2265.1980.tb02131.x. PMID 6772356. S2CID 5989414.
- ^ Goh HH, Ratnam SS (October 1990). "Effect of estrogens on prolactin secretion in transsexual subjects". Arch Sex Behav. 19 (5): 507–16. doi:10.1007/BF02442351. PMID 2260915. S2CID 39940587.
- ^ a b c Düsterberg B, Schmidt-Gollwitzer M, Hümpel M (1985). "Pharmacokinetics and biotransformation of estradiol valerate in ovariectomized women". Horm. Res. 21 (3): 145–54. doi:10.1159/000180039. PMID 2987096.
- ^ a b c d Gahart BL, Nazareno AR, Ortega M (9 May 2018). Gahart's 2019 Intravenous Medications - E-Book: A Handbook for Nurses and Health Professionals. Elsevier Health Sciences. pp. 361–. ISBN 978-0-323-61273-9.
- ^ Bhavnani BR, Stanczyk FZ (July 2014). "Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action". J. Steroid Biochem. Mol. Biol. 142: 16–29. doi:10.1016/j.jsbmb.2013.10.011. PMID 24176763. S2CID 1360563.
- ^ a b c "Premarin Intravenous (conjugated estrogens, USP) for injection" (PDF). Wyeth Pharmaceuticals, Inc. U.S. Food and Drug Administration. November 2017.
- ^ a b Bergenheim AT, Henriksson R (February 1998). "Pharmacokinetics and pharmacodynamics of estramustine phosphate". Clin Pharmacokinet. 34 (2): 163–72. doi:10.2165/00003088-199834020-00004. PMID 9515186. S2CID 1943973.
- ^ a b Scoccia B, Demir H, Elter K, Scommegna A (2009). "Successful medical management of post-hysteroscopic metroplasty bleeding with intravenous estrogen therapy: a report of two cases and review of the literature". J Minim Invasive Gynecol. 16 (5): 639–42. doi:10.1016/j.jmig.2009.05.012. PMID 19835811.
- ^ a b c Gunnarsson PO, Forshell GP (June 1984). "Clinical pharmacokinetics of estramustine phosphate". Urology. 23 (6 Suppl): 22–7. doi:10.1016/S0090-4295(84)80093-X. PMID 6375076.
- ^ Loeser AA (February 1948). "The action of intravenously injected sex hormones and other substances on the blood flow in the human endometrium". J Obstet Gynaecol Br Emp. 55 (1): 17–22. doi:10.1111/j.1471-0528.1948.tb07044.x. PMID 18902558. S2CID 72219242.
- ^ Hertz R, Tullner WW (October 1949). "Intravenous administration of massive dosages of estrogen to the human subject; blood levels attained". Proc. Soc. Exp. Biol. Med. 72 (1): 187–91. doi:10.3181/00379727-72-17373. PMID 15391710. S2CID 9223167.
- ^ Tullner WW, Young JP, Hertz R (1952). "Administration of Massive Dosage of Oestrogen to Breast and Prostatic Cancer Patients; Blood Levels Attained". Ciba Foundation Symposium - Steroid Hormones and Tumour Growth, Book I of Colloquia on Endocrinology. Novartis Foundation Symposia. Vol. 1. John Wiley & Sons. pp. 157–169. doi:10.1002/9780470718759.ch13. ISBN 978-0-470-71875-9. ISSN 1935-4657.
- ^ McManus JM, Bohn K, Alyamani M, Chung YM, Klein EA, Sharifi N (2019). "Rapid and structure-specific cellular uptake of selected steroids". PLOS ONE. 14 (10): e0224081. Bibcode:2019PLoSO..1424081M. doi:10.1371/journal.pone.0224081. PMC 6797172. PMID 31622417.
- ^ Gray JM, Dudley SD, Wade GN (January 1981). "In vivo cell nuclear binding of 17 beta-[3H]estradiol in rat adipose tissues". Am. J. Physiol. 240 (1): E43–6. doi:10.1152/ajpendo.1981.240.1.E43. PMID 7457597.
- ^ Mendel CM (August 1989). "The free hormone hypothesis: a physiologically based mathematical model". Endocr. Rev. 10 (3): 232–74. doi:10.1210/edrv-10-3-232. PMID 2673754.
- ^ a b c Dorfman RI (1961). "Steroid Hormone Metabolism". Radioactive Isotopes in Physiology Diagnostics and Therapy / Künstliche Radioaktive Isotope in Physiologie Diagnostik und Therapie. Springer. pp. 1223–1241. doi:10.1007/978-3-642-49761-2_39. ISBN 978-3-642-49477-2.
- ^ a b Sandberg AA, Slaunwhite WR (August 1957). "Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women". J. Clin. Invest. 36 (8): 1266–78. doi:10.1172/JCI103524. PMC 1072719. PMID 13463090.
Further reading
[edit]- Wolf AS, Schneider HP (1989). Östrogene in Diagnostik und Therapie [Estrogens in Diagnostics and Therapy]. Springer-Verlag. pp. 1–. ISBN 978-3-642-75101-1.
- Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-O. PMID 2170822.
- Lobo RA, Cassidenti DL (January 1992). "Pharmacokinetics of oral 17 beta-estradiol". J Reprod Med. 37 (1): 77–84. PMID 1548642.
- O'Connell MB (September 1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9S): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713. S2CID 10159196.
- Oettel M, Schillinger E (1999). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. ISBN 978-3-642-58616-3.
- Oettel M, Schillinger E (1999). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. ISBN 978-3-642-60107-1.
- Kuhl H (2000). "Pharmacology of estradiol and estriol". Menopause Review. 5: 23–44.
- Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". J Midwifery Womens Health. 47 (3): 130–8. doi:10.1016/S1526-9523(02)00233-7. PMID 12071379.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Barnes RB, Levrant SG (2007). "Pharmacology of Estrogens". Treatment of the Postmenopausal Woman. Academic Press. pp. 767–777. doi:10.1016/B978-012369443-0/50066-1. ISBN 978-0-12-369443-0.
- Fruzzetti F, Trémollieres F, Bitzer J (2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- Stanczyk FZ, Archer DF, Bhavnani BR (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–27. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.










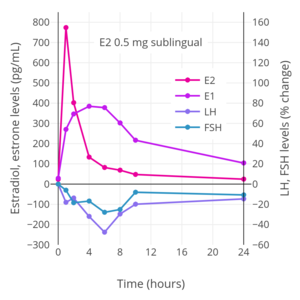





![Levels of estradiol at steady-state over a period of 4 days with different dosages of Vivelle-type (Vivelle, Vivelle-Dot, Mylan generic) twice-weekly transdermal estradiol matrix patches applied to the abdomen and worn until day 4 in postmenopausal women.[226][227][228]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d9/Estradiol_levels_with_different_dosages_of_transdermal_estradiol_patches_%28Vivelle-type%29_in_postmenopausal_women.png/300px-Estradiol_levels_with_different_dosages_of_transdermal_estradiol_patches_%28Vivelle-type%29_in_postmenopausal_women.png)
![Levels of estradiol over a period of 7.5 days after a single application of different dosages of a Climara-type (Climara, Menostar, Mylan generic) once-weekly transdermal estradiol matrix patch to the abdomen and removed on day 7 in postmenopausal women.[229][230][231]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/43/Estradiol_levels_with_different_dosages_of_transdermal_estradiol_patches_%28Climara-type%29_in_postmenopausal_women.png/300px-Estradiol_levels_with_different_dosages_of_transdermal_estradiol_patches_%28Climara-type%29_in_postmenopausal_women.png)
![Levels of estradiol over a period of 8 days after a single application of a 50 or 100 μg/day Climara-type (Climara, Menostar, Mylan generic) once-weekly transdermal estradiol matrix patch to the abdomen and removed on day 7 in postmenopausal women.[232]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c1/Estradiol_levels_with_a_50_or_100_%CE%BCg_per_day_transdermal_estradiol_patch_%28Climara-type%29_in_women.png/271px-Estradiol_levels_with_a_50_or_100_%CE%BCg_per_day_transdermal_estradiol_patch_%28Climara-type%29_in_women.png)
![Levels of estradiol and estrone with application of a single 50 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[188]](http://upload.wikimedia.org/wikipedia/commons/thumb/5/5b/Estrogen_levels_with_a_50_%C2%B5g_per_day_estradiol_transdermal_patch_in_postmenopausal_women.png/300px-Estrogen_levels_with_a_50_%C2%B5g_per_day_estradiol_transdermal_patch_in_postmenopausal_women.png)
![Estradiol level with a single 100 µg/day estradiol reservoir patch (Estraderm) with and without ethanol added in postmenopausal women.[17][233] This patch has a 3- to 4-day duration and is designed for twice-weekly application. In one group, ethanol was injected into the area between the patch and the skin on day 3.[17][233] This gave significantly higher and prolonged estradiol levels.[17][233]](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e1/Estradiol_levels_with_a_single_application_of_a_100_%C2%B5g_per_day_estradiol_reservoir_patch_%28Estraderm%29_with_and_without_an_ethanol_injection_in_postmenopausal_women.png/299px-thumbnail.png)
![Estradiol and testosterone levels with high-dosage transdermal estradiol in the form of two to six 100 µg/day estradiol patches (Progynova TS forte) in men with prostate cancer.[14][214][234]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/ab/Estradiol_and_testosterone_levels_with_high-dose_estradiol_patches_in_men.png/300px-Estradiol_and_testosterone_levels_with_high-dose_estradiol_patches_in_men.png)
![Estradiol levels with 50 to 100 μg/day transdermal estradiol patches applied to the forearm and to the scrotum in a crossover study in 2 men with prostate cancer.[204] In 35 men treated continuously with one 100 μg/day estradiol patch scrotally, the mean estradiol level was ~500 pg/mL (range ~125–1,200 pg/mL).[204]](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e9/Estradiol_levels_with_transdermal_estradiol_patches_applied_to_the_forearm_or_scrotum_in_men.png/300px-Estradiol_levels_with_transdermal_estradiol_patches_applied_to_the_forearm_or_scrotum_in_men.png)
![Levels of estradiol and estrone with once daily application of 1.25 g of a transdermal estradiol gel (EstroGel) containing 0.06% or 0.75 mg estradiol after 14 days of continuous therapy in postmenopausal women.[194]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b9/Estrogen_levels_with_1.25_g_of_0.06%25_transdermal_estradiol_gel_%28EstroGel%29_per_day_in_postmenopausal_women.png/289px-Estrogen_levels_with_1.25_g_of_0.06%25_transdermal_estradiol_gel_%28EstroGel%29_per_day_in_postmenopausal_women.png)
![Levels of estradiol with once daily application of a transdermal estradiol gel (EstroGel) containing 1.5 or 3.0 mg estradiol over 3 days of administration in postmenopausal women.[96][249]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/3c/Estradiol_levels_with_1.5_or_3.0_mg_per_day_transdermal_estradiol_gel_in_postmenopausal_women.png/299px-Estradiol_levels_with_1.5_or_3.0_mg_per_day_transdermal_estradiol_gel_in_postmenopausal_women.png)
![Estradiol levels after the last dose with 1 mg/day transdermal estradiol gel applied to different amounts of skin area (200 cm2, 400 cm2, or as large as possible) in postmenopausal women.[250]](http://upload.wikimedia.org/wikipedia/commons/thumb/1/19/Estradiol_levels_with_1_mg_per_day_transdermal_estradiol_gel_applied_to_different_amounts_of_area_in_postmenopausal_women.png/262px-Estradiol_levels_with_1_mg_per_day_transdermal_estradiol_gel_applied_to_different_amounts_of_area_in_postmenopausal_women.png)
![Estrogen levels with a single vaginal application of 0.5 mg micronized estradiol in 2 mL solution in postmenopausal women.[257][258][259]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/63/Estrogen_levels_with_a_single_vaginal_application_of_0.5_mg_estradiol_in_postmenopausal_women.png/300px-Estrogen_levels_with_a_single_vaginal_application_of_0.5_mg_estradiol_in_postmenopausal_women.png)
![Percent change in estradiol, estrone, LH, and FSH levels with a single vaginal application of 1 mg micronized estradiol in saline in hypoestrogenic women.[260][40][111]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4d/Estrogen_and_gonadotropin_levels_with_1_mg_vaginal_estradiol_in_women.png/300px-Estrogen_and_gonadotropin_levels_with_1_mg_vaginal_estradiol_in_women.png)
![Tritiated estradiol radioactivity in blood with a single intramuscular injection of 1.5 to 2.8 μg tritiated estradiol in aqueous solution in four women.[324] Peak blood radioactivity occurred within 15 minutes in three of the women and in the remaining woman after 2 hours.[324] Source: Davis et al. (1963).[324]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/27/Tritiated_estradiol_blood_radioactivity_with_a_single_intramuscular_injection_of_1.5_to_2.8_mcg_tritiated_estradiol_in_aqueous_solution_in_women.png/296px-Tritiated_estradiol_blood_radioactivity_with_a_single_intramuscular_injection_of_1.5_to_2.8_mcg_tritiated_estradiol_in_aqueous_solution_in_women.png)
![Estradiol and testosterone levels with a single intramuscular injection of 2 mg estradiol in an aqueous preparation in healthy young men.[325] Type of aqueous preparation (solution or suspension) was not specified.[325] Source: Jones et al. (1978).[325]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/86/Estradiol_and_testosterone_levels_with_a_single_intramuscular_injection_of_2_mg_aqueous_estradiol_in_healthy_young_men.png/300px-Estradiol_and_testosterone_levels_with_a_single_intramuscular_injection_of_2_mg_aqueous_estradiol_in_healthy_young_men.png)
![Estradiol levels after a single intramuscular injection of 5 mg estradiol benzoate, 5 mg estradiol valerate, or 5 mg estradiol cypionate in oil solution in women.[274] Source: Oriowo et al. (1980).[274]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/33/Estradiol_levels_after_a_single_5_mg_intramuscular_injection_of_estradiol_esters.png/300px-Estradiol_levels_after_a_single_5_mg_intramuscular_injection_of_estradiol_esters.png)
![Simplified curves of estradiol levels after intramuscular injection of different 5 mg estradiol benzoate, 5 mg estradiol valerate, 5 mg estradiol cypionate, or 10 mg estradiol enanthate in oil solution in women.[326] Source: Garza-Flores (1994).[326]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8c/Idealized_curves_of_estradiol_levels_after_injection_of_different_estradiol_esters_in_women.png/279px-Idealized_curves_of_estradiol_levels_after_injection_of_different_estradiol_esters_in_women.png)
![Vaginal cornification with a single intramuscular injection of 5 mg estradiol benzoate, 5 mg estradiol dipropionate, or 5 to 25 mg estradiol cypionate in oil solution in women.[327] Source: Schwartz & Soule (1955).[327]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/23/Vaginal_cornification_with_a_single_intramuscular_injection_of_different_estradiol_esters_in_women.png/300px-Vaginal_cornification_with_a_single_intramuscular_injection_of_different_estradiol_esters_in_women.png)
![Estradiol levels after a short intravenous infusion of 20 mg estradiol in aqueous solution or an intramuscular injection of an equimolar dose of estradiol benzoate, estradiol valerate, or estradiol undecylate in oil solution in women.[328][280] Sources: Geppert (1975) and Leyendecker et al. (1975).[328][280]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b1/Estradiol_levels_after_injections_of_estradiol%2C_estradiol_benzoate%2C_estradiol_valerate%2C_and_estradiol_undecylate_in_women.png/300px-Estradiol_levels_after_injections_of_estradiol%2C_estradiol_benzoate%2C_estradiol_valerate%2C_and_estradiol_undecylate_in_women.png)
![Estradiol levels after a single intramuscular injection of 10 mg estradiol valerate or 100 mg estradiol undecylate in oil solution.[329] Source: Vermeulen (1975).[329]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/65/Estradiol_levels_after_a_single_intramuscular_injection_of_10_mg_estradiol_valerate_and_100_mg_estradiol_undecylate.png/300px-Estradiol_levels_after_a_single_intramuscular_injection_of_10_mg_estradiol_valerate_and_100_mg_estradiol_undecylate.png)
![Estradiol and testosterone levels after a single intramuscular injection of 320 mg polyestradiol phosphate in aqueous solution in men with prostate cancer.[320] Source: Stege et al. (1996).[320]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7d/Estradiol_and_testosterone_levels_with_a_single_intramuscular_injection_of_320_mg_polyestradiol_phosphate_in_men.png/300px-Estradiol_and_testosterone_levels_with_a_single_intramuscular_injection_of_320_mg_polyestradiol_phosphate_in_men.png)
![Estradiol levels after the fourth dose during continuous therapy with estradiol and progesterone microspheres in aqueous suspension by intramuscular injection once per month in menopausal women.[330][331] Source: Espino y Sosa et al. (2019).[330]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/42/Estradiol_levels_with_estradiol_and_progesterone_microspheres_by_intramuscular_injection_once_per_month_in_menopausal_women.png/268px-Estradiol_levels_with_estradiol_and_progesterone_microspheres_by_intramuscular_injection_once_per_month_in_menopausal_women.png)
![Baseline-corrected levels of estradiol, estrone, and estrone conjugates (e.g., estrone sulfate) after a single intravenous infusion of 0.3 mg estradiol in aqueous solution in women.[60]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/db/Estrogen_levels_after_a_single_intravenous_injection_of_0.3_mg_estradiol_in_premenopausal_women.png/331px-Estrogen_levels_after_a_single_intravenous_injection_of_0.3_mg_estradiol_in_premenopausal_women.png)
![Estradiol levels after a short intravenous infusion of 20 mg estradiol in aqueous solution or an intramuscular injection of an equimolar dose of estradiol benzoate, estradiol valerate, or estradiol undecylate in oil solution in women.[281][280] Earlier time points than 12 hours post-infusion for intravenous estradiol were not measured.[281][280]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b1/Estradiol_levels_after_injections_of_estradiol%2C_estradiol_benzoate%2C_estradiol_valerate%2C_and_estradiol_undecylate_in_women.png/375px-Estradiol_levels_after_injections_of_estradiol%2C_estradiol_benzoate%2C_estradiol_valerate%2C_and_estradiol_undecylate_in_women.png)
