From Wikipedia, the free encyclopedia
Chemical compound
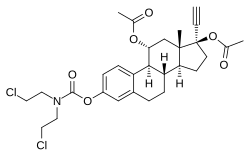
Cytestrol acetate Other names 11α-Hydroxyethinylestradiol 3-(bis(2-chloroethyl)carbamate) 11α,17β-diacetate; 17α-Ethynylestra-1,3,5(10)-triene-3,11α,17β-triol 11α,17β-diacetate 3-(bis(2-chloroethyl)carbamate)
(1S ,10S ,11S ,14R ,15S ,17R )-17-(Acetyloxy)-5-{[bis(2-chloroethyl)carbamoyl]oxy}-14-ethynyl-15-methyltetracyclo[8.7.0.02,7 .011,15 ]heptadeca-2,4,6-trien-14-yl acetate
PubChem CID ChemSpider Formula C 29 H 35 Cl 2 N O 6 Molar mass −1 3D model (JSmol )
[H][C@@]12CC[C@@](OC(C)=O)(C#C)[C@@]1(C)C[C@@H](OC(C)=O)[C@]1([H])C3=CC=C(OC(=O)N(CCCl)CCCl)C=C3CC[C@@]21[H]
InChI=1S/C29H35Cl2NO6/c1-5-29(38-19(3)34)11-10-24-23-8-6-20-16-21(37-27(35)32(14-12-30)15-13-31)7-9-22(20)26(23)25(36-18(2)33)17-28(24,29)4/h1,7,9,16,23-26H,6,8,10-15,17H2,2-4H3/t23-,24-,25+,26+,28-,29-/m0/s1
Key:RRJRVDYYFLBGSE-UPRLNUCLSA-N
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent (i.e., chemotherapeutic ) which was developed for the treatment of breast cancer but was never marketed.[ 1] [ 2] [ 3] [ 4]
It is an 11α-hydroxylated derivative of ethinylestradiol in which a bis(2-chloroethyl)amine nitrogen mustard moiety has been attached as an ester at the C3 position and acetate esters have been attached at the C11α and C17β positions.[ 1] [ 2] [ 3] [ 4] mechanism of action of cytestrol acetate in breast cancer is two-fold: (1) acting as an antiestrogen similarly to fulvestrant or ICI-164384 ; and (2) having cytostatic actions via the carbamate–nitrogen mustard moiety analogously to estramustine phosphate .[ 1] [ 2] [ 3] [ 4] efficacy against breast cancer superior to that of tamoxifen in in vitro [ 1] [ 2] [ 3]
^ a b c d Oborotov AV, Smirnova ZS, Osetrova IP, Polozkova AP, Rzheznikov VM (1999). "Antitumor activity of various medicinal forms of the new estrogenocytostatic drug cytestrol acetate". Pharmaceutical Chemistry Journal . 33 (10): 526–527. doi :10.1007/BF02508372 . ISSN 0091-150X . S2CID 5550495 . ^ a b c d Smirnova ZS, Rzheznikov VM, Tolkachev VN, Borisova LM, Kiseleva MP, Semeĭkin AV, et al. (2014). "[Antitumor and antiproliferative action of the steroidal cytostatic antiestrogen cytestrol acetate on hormone-dependent tumor models]". Eksperimental'naia i Klinicheskaia Farmakologiia (in Russian). 77 (10): 31–35. PMID 25518525 . ^ a b c d Smirnova ZS (2003). "[Experimental Study of Hormonocytostatics for Treatment of Breast Cancer.]" [Russian biotherapeutic journal]. Российский биотерапевтический журнал (in Russian). 2 (2). ^ a b c Smirnova ZS, Rzheznikov VM, Tolkachev VN, Borisova LM, Kiseleva MP, Semeykin AV, Fedocheva TA, Shirokikh KE, Banin VV, Shimanovsky NL (November 2014). "Противоопухолевое и антипролиферативное действие стероидного антиэстрогена цитэстрола ацетата на моделях гормонозависимых опухолей" [Antitumor and antiproliferative effects of the steroid antiestrogen citestrol acetate in models of hormone-dependent tumors.]. Экспериментальная и клиническая фармакология [Experimental and clinical pharmacology. ] (in Russian). 77 (10): 31–35.
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown