Tetrahydrofuran (data page)
Appearance
This page provides supplementary chemical data on tetrahydrofuran.
Material Safety Data Sheet
[edit]The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as SIRI, and follow its directions. MSDS is available at Mallinckrodt Baker.
Structure and properties
[edit]| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.4040 at 25°C |
| Abbe number | ? |
| Dielectric constant, εr[1] | 7.52 ε0 at 22 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Viscosity[1] | 0.456 mPa·s at 25°C |
Thermodynamic properties
[edit]| Phase behavior | |
|---|---|
| Triple point | 164.76 K (−108.39 °C), ? Pa |
| Critical point | 541 K (268 °C), 5190 kPa |
| Std enthalpy change of fusion, ΔfusH |
8.540 kJ/mol |
| Std entropy change of fusion, ΔfusS |
51.8 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
32 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
51.8 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp[2] | 81.65 J/(mol K) at −108.39°C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
203.8 J/(mol K) |
| Enthalpy of combustion, ΔcH |
−2501.2 kJ/mol |
| Heat capacity, cp[2] | 107.4 J/(mol K) at −108.39 °C
123.9 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−184.2 kJ/mol |
| Standard molar entropy, S |
301.7 J/(mol K) |
| Heat capacity, cp | 76.6 J/(mol K) at 25°C |
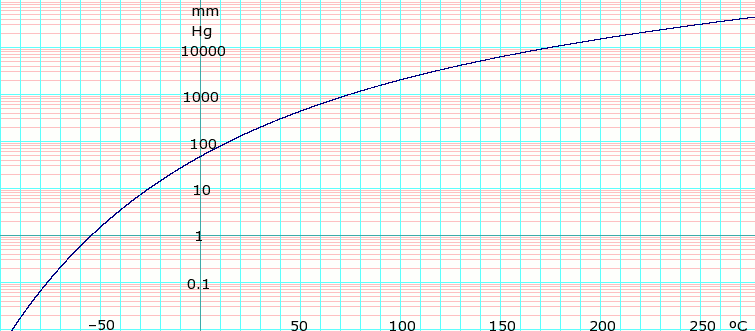
Vapor pressure of liquid
[edit]Vapor pressure 143 mm Hg at 20°C[1]
| P in mBar[3] | 9.9 | 19.5 | 36.3 | 63.9 | 107 | 173 | 268 | 402 | 586 | 831 | 1013 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T in °C | −30 | −20 | −10 | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 66 | |
Distillation data
[edit]| Vapor-Liquid Equilibrium of Tetrahydrofuran/Ethanol[5] P = 100 kPa | ||
| BP Temp. °C |
% by mole THF | |
|---|---|---|
| liquid | vapor | |
| 78. | 0.00 | 0.00 |
| 77.4 | 1.72 | 3.69 |
| 76.2 | 5.36 | 11.5 |
| 73.8 | 13.9 | 26.4 |
| 71.0 | 26.3 | 42.8 |
| 67.7 | 49.7 | 62.8 |
| 67.2 | 54.3 | 65.4 |
| 65.9 | 71.5 | 76.2 |
| 65.4 | 85.7 | 86.5 |
| 65.2 | 90.8 | 90.8 |
| 65.4 | 91.8 | 91.48 |
| 65.4 | 94.99 | 94.46 |
| 65.5 | 98.15 | 97.9 |
| 65.6 | 100.0 | 100.0 |
Spectral data
[edit]| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
This box:
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References
[edit]- ^ a b c "Tetrahydrofuran Physical Properties". Sigma-Aldrich. Retrieved 25 June 2009.
- ^ a b Lebedev, B.V; Rabinovich, I.B; Milov, V.I; Lityagov, V.Ya (1 April 1978). "Thermodynamic properties of tetrahydrofuran from 8 to 322 K". The Journal of Chemical Thermodynamics. 10 (4): 321–329. doi:10.1016/0021-9614(78)90064-2. ISSN 0021-9614.
- ^ "Tetrahydrofuran (THF) Storage and Handling" (PDF). BASF. Archived from the original (PDF) on 9 March 2008. Retrieved 24 May 2007.
- ^ "Pure Component Properties". Chemical Engineering Research Information Center. Retrieved 24 May 2007.
- ^ "Binary Vapor-Liquid Equilibrium Data". Chemical Engineering Research Information Center. Retrieved 24 May 2007.
- Linstrom, Peter (1997). "NIST Standard Reference Database". National Institute of Standards and Technology. doi:10.18434/T4D303.
{{cite journal}}: Cite journal requires|journal=(help)


