From Wikipedia, the free encyclopedia
|type= vaccine/combo/mab/blank
|
|
|
Infobox drug/sandbox (edit · t · history · diff · links · /test · Source · e · t · hist · links · /subpages · /doc · /doc edit)
- /testcases2 -- titles, licence, EMA
- /testcases3 -- pregcat, legal, licence, PLLR, ATC; Wikidata
- /testcases4 -- chem formula, mab
- /testcases5 -- identifiers, second id's
- /testcases6 -- all up
- /testcases7images -- images
- /testcases8 -- type, titles
- /testcases9 -- order variants, container_only
- /testcases10 -- pharmacokinetic, localINN (2017) has (data page) -- is a redirect
- /testcases11 -- hormone, gene therapy (2018), has (data page)
- /testcases-FDA -- FDA 2023
- /testcases-warning -- warning box(es)
|
- Purge mab = data10; source=data11, target=data12 (freetext)
| mab = Whole antibody
| fab = [[Fab fragment]]
| f(ab')2 = [[F(ab')2 fragment|F(ab')<sub>2</sub> fragment]]
| fab' = [[Fab' fragment]]
| scfv = [[Single-chain variable fragment]]
| discfv = Di-[[single-chain variable fragment]]
| sdab = [[Single domain antibody]]
| 3funct = [[Trifunctional antibody]]
| clfab = [[Chemically linked Fab]]
| bite = [[Bi-specific T-cell engager]]
| ?
- if mab= then use source=
| label11 = [[Monoclonal antibody#Production|Source]]
| data11 = {{#ifeq: {{{type|}}} | mab | {{#switch: {{lc:{{{source|}}}}}
| =
| a = [[Rat]]
| e = [[Hamster]]
| i = [[Primate]]
| o = [[Mouse]]
| u = [[Human]]
| xi/a = [[chimeric antibody|Chimeric]] ([[rat]]/[[human]])
| xi/e = [[chimeric antibody|Chimeric]] ([[hamster]]/[[human]])
| xi/i = [[chimeric antibody|Chimeric]] ([[primate]]/[[human]])
| xi/o = [[chimeric antibody|Chimeric]] ([[mouse]]/[[human]])
| xi = [[chimeric antibody|Chimeric]]
| zu/a = [[Humanized]] (from [[rat]])
| zu/e = [[Humanized]] (from [[hamster]])
| zu/i = [[Humanized]] (from [[primate]])
| zu/o = [[Humanized]] (from [[mouse]])
| zu = [[Humanized]]
| xizu/a = [[chimeric antibody|Chimeric]]/[[humanized]] hybrid ([[rat]]/[[human]])
| xizu/e = [[chimeric antibody|Chimeric]]/[[humanized]] hybrid ([[hamster]]/[[human]])
| xizu/i = [[chimeric antibody|Chimeric]]/[[humanized]] hybrid ([[primate]]/[[human]])
| xizu/o = [[chimeric antibody|Chimeric]]/[[humanized]] hybrid ([[mouse]]/[[human]])
| xizu = [[chimeric antibody|Chimeric]]/[[humanized]] hybrid
| axo = [[Rat]]/[[mouse]] hybrid
| {{{source|}}} [[:Category:Drugboxes with unformatted antibody source]]
|
|vaccin_type=
|killed |inactivated = Killed/Inactivated
|attenuated = [[Attenuated virus]]
|live = Live bacteria
|toxoid = [[Toxoid]]
|subunit = Subunit
|protein subunit |protein = [[Protein subunit]]
|conjugate=[[Conjugate vaccine]]
|recombinant = [[Recombinant DNA|Recombinant Vector]]
|dna = [[DNA vaccination]]
| ?
TITLE tests (drug_name, INN)
[edit]Purge
Side by side comparison| {{Infobox drug}} | {{Infobox drug/sandbox}} |
|---|
Infobox drug/testcases8
INN: Linezolid | 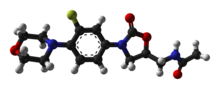 | |
| Pronunciation | |
|---|
| Trade names | Linospan, Zyvox, Zyvoxam, Zyvoxid |
|---|
| Other names | Lenzomore |
|---|
| AHFS/Drugs.com | Monograph |
|---|
| MedlinePlus | a602004 |
|---|
| License data |
|
|---|
Pregnancy
category | |
|---|
Dependence
liability | High |
|---|
Addiction
liability | Low |
|---|
Routes of
administration | Intravenous infusion, oral |
|---|
| ATC code | |
|---|
|
| Bioavailability | ~100% (oral) |
|---|
| Protein binding | Low (31%) |
|---|
| Metabolism | Hepatic (50–70%, CYP not involved) |
|---|
| Metabolites | some stuff |
|---|
| Onset of action | 1 hr |
|---|
| Elimination half-life | 4.2–5.4 hours (shorter in children) |
|---|
| Duration of action | 1 to 3 hr |
|---|
| Excretion | Nonrenal, renal, and fecal |
|---|
|
(S)-N-({3-[3-fluoro-4-(morpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)acetamide
| | CAS Number | |
|---|
| PubChem CID | |
|---|
| IUPHAR/BPS | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| KEGG | |
|---|
| ChEMBL | |
|---|
| NIAID ChemDB | |
|---|
| PDB ligand | |
|---|
|
| Formula | C16H20FN3O4 |
|---|
| Molar mass | 337.346 g/mol g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
| Density | 1.40 g/cm3 |
|---|
| Melting point | 135 °C (275 °F) |
|---|
| Boiling point | 140 °C (284 °F) (decomposes) |
|---|
| Solubility in water | 3 mg/mL (20 °C) |
|---|
O=C1O[C@@H](CNC(=O)C)CN1c3cc(F)c(N2CCOCC2)cc3
|
InChI=1S/C16H20FN3O4/c1-11(21)18-9-13-10-20(16(22)24-13)12-2-3-15(14(17)8-12)19-4-6-23-7-5-19/h2-3,8,13H,4-7,9-10H2,1H3,(H,18,21)/t13-/m0/s1  Y YKey:TYZROVQLWOKYKF-ZDUSSCGKSA-N  Y Y
|  N N Y (what is this?) (verify) Y (what is this?) (verify) | |
Infobox drug/testcases8
INN: Linezolid |  | |
| Pronunciation | |
|---|
| Trade names | Linospan, Zyvox, Zyvoxam, Zyvoxid |
|---|
| Other names | Lenzomore |
|---|
| AHFS/Drugs.com | Monograph |
|---|
| MedlinePlus | a602004 |
|---|
| License data |
|
|---|
Pregnancy
category | |
|---|
Dependence
liability | High |
|---|
Addiction
liability | Low |
|---|
Routes of
administration | Intravenous infusion, oral |
|---|
| ATC code | |
|---|
|
| Bioavailability | ~100% (oral) |
|---|
| Protein binding | Low (31%) |
|---|
| Metabolism | Hepatic (50–70%, CYP not involved) |
|---|
| Metabolites | some stuff |
|---|
| Onset of action | 1 hr |
|---|
| Elimination half-life | 4.2–5.4 hours (shorter in children) |
|---|
| Duration of action | 1 to 3 hr |
|---|
| Excretion | Nonrenal, renal, and fecal |
|---|
|
(S)-N-({3-[3-fluoro-4-(morpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)acetamide
| | CAS Number | |
|---|
| PubChem CID | |
|---|
| IUPHAR/BPS | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| KEGG | |
|---|
| ChEMBL | |
|---|
| NIAID ChemDB | |
|---|
| PDB ligand | |
|---|
|
| Formula | C16H20FN3O4 |
|---|
| Molar mass | 337.346 g/mol g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
| Density | 1.40 g/cm3 |
|---|
| Melting point | 135 °C (275 °F) |
|---|
| Boiling point | 140 °C (284 °F) (decomposes) |
|---|
| Solubility in water | 3 mg/mL (20 °C) |
|---|
O=C1O[C@@H](CNC(=O)C)CN1c3cc(F)c(N2CCOCC2)cc3
|
InChI=1S/C16H20FN3O4/c1-11(21)18-9-13-10-20(16(22)24-13)12-2-3-15(14(17)8-12)19-4-6-23-7-5-19/h2-3,8,13H,4-7,9-10H2,1H3,(H,18,21)/t13-/m0/s1  Y YKey:TYZROVQLWOKYKF-ZDUSSCGKSA-N  Y Y
|  N N Y (what is this?) (verify) Y (what is this?) (verify) | |
Purge
English language variants: Licence or License
[edit]- See also WP:ENGVAR: english language variant of the article
- In {{Infobox drug}}: spelling of licence/license. Default is to be en-US: License.
- Use new parameter
|engvar= for non-default spelling:
- Default licenSe (US)
|engvar=en-US licenSe
|engvar=en-UK licenCe
|engvar=en-NZ licenCe
The infobox title is a logical result of input:
- {{PAGENAME}} (default)
|drug_name= (overwrites, eg when PAGENAME is not the INN drugname)|INN= (added, when PAGENAME/drug_name is not the INN name)
- See Category:Infobox drug articles with non-default infobox title (837)
Purge (reduced infobox)
- Current infobox title: Diamorphine (INN)
- The new situation required an edit.
Side by side comparison| {{Infobox drug}} | {{Infobox drug/sandbox}} |
|---|
Heroin
INN: Diamorphine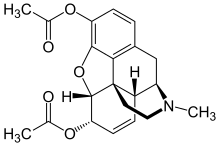 | 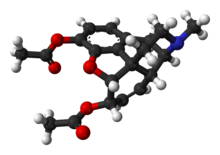 | |
| Pronunciation | |
|---|
| Other names | Diamorphine, Diacetylmorphine, Acetomorphine, (Dual) Acetylated morphine, Morphine diacetate |
|---|
| AHFS/Drugs.com | heroin |
|---|
Dependence
liability | Physical: Very high
Psychological: Very high |
|---|
Addiction
liability | Very high |
|---|
Routes of
administration | Inhalation, transmucosal, intravenous, oral, intranasal, rectal, intramuscular |
|---|
| ATC code | |
|---|
|
| Legal status |
- AU: S9 (Prohibited substance)
|
|---|
|
(5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol diacetate
| | CAS Number | |
|---|
| PubChem CID | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEBI | |
|---|
| ChEMBL | |
|---|
|
| Formula | C21H23NO5 |
|---|
| Molar mass | 369.41 g/mol g·mol−1 |
|---|
|
Heroin
INN: Diamorphine |  | |
| Pronunciation | |
|---|
| Other names | Diamorphine, Diacetylmorphine, Acetomorphine, (Dual) Acetylated morphine, Morphine diacetate |
|---|
| AHFS/Drugs.com | heroin |
|---|
Dependence
liability | Physical: Very high
Psychological: Very high |
|---|
Addiction
liability | Very high |
|---|
Routes of
administration | Inhalation, transmucosal, intravenous, oral, intranasal, rectal, intramuscular |
|---|
| ATC code | |
|---|
|
| Legal status |
- AU: S9 (Prohibited substance)
|
|---|
|
(5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol diacetate
| | CAS Number | |
|---|
| PubChem CID | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEBI | |
|---|
| ChEMBL | |
|---|
|
| Formula | C21H23NO5 |
|---|
| Molar mass | 369.41 g/mol g·mol−1 |
|---|
|
Purge Reduced infobox.
- Current infobox title: Lysergic acid diethylamide (pagename, not INN)
- New situation would be (adding "(LSD)" is just a liberty I took, to help our readers):
Side by side comparison| {{Infobox drug}} | {{Infobox drug/sandbox}} |
|---|
Lysergic acid diethylamide (LSD)
INN: Lysergide | |
| Other names | Acid, LSD, lysergide |
|---|
Dependence
liability | Low |
|---|
Addiction
liability | None |
|---|
Routes of
administration | Oral, etc |
|---|
| ATC code | |
|---|
|
| Legal status |
|
|---|
|
| Metabolism | Hepatic |
|---|
| Elimination half-life | 3–5 hours |
|---|
| Excretion | Renal |
|---|
|
(6aR,9R)-N,N-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindolo-[4,3-fg]quinoline-9-carboxamide
| | CAS Number | |
|---|
| PubChem CID | |
|---|
| IUPHAR/BPS | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEBI | |
|---|
| ChEMBL | |
|---|
|
| Formula | C20H25N3O |
|---|
| Molar mass | 323.440 g·mol−1 |
|---|
|
Lysergic acid diethylamide (LSD)
INN: Lysergide | |
| Other names | Acid, LSD, lysergide |
|---|
Dependence
liability | Low |
|---|
Addiction
liability | None |
|---|
Routes of
administration | Oral, etc |
|---|
| ATC code | |
|---|
|
| Legal status |
|
|---|
|
| Metabolism | Hepatic |
|---|
| Elimination half-life | 3–5 hours |
|---|
| Excretion | Renal |
|---|
|
(6aR,9R)-N,N-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindolo-[4,3-fg]quinoline-9-carboxamide
| | CAS Number | |
|---|
| PubChem CID | |
|---|
| IUPHAR/BPS | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEBI | |
|---|
| ChEMBL | |
|---|
|
| Formula | C20H25N3O |
|---|
| Molar mass | 323.440 g·mol−1 |
|---|
|
- No need to use
|INN=. Note the titletext (mousehover).






