Rubidium pertechnetate
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| O4RbTc | |
| Molar mass | 247 g·mol−1 |
| Appearance | white solid[1] |
| 1.167 g/100mL[1] | |
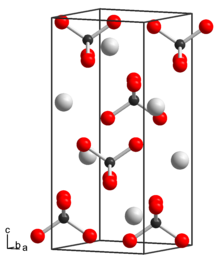
| Structure | |
| tetragonal | |
| I41/a | |
a = 576.2 pm, c = 1354.3 pm
| |
| Related compounds | |
Other anions
|
Rubidium perchlorate Rubidium permanganate |
Other cations
|
Pertechnate Lithium pertechnetate Sodium pertechnetate Potassium pertechnetate Caesium pertechnetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rubidium pertechnetate is a pertechnetate of rubidium, with the chemical formula RbTcO4.
Preparation
[edit]Rubidium pertechnetate can be prepared by reacting rubidium carbonate with ammonium pertechnetate:[2]
Rb2CO3 + 2 NH4TcO4 → 2 RbTcO4 + (NH4)2CO3
Properties
[edit]Rubidium pertechnetate crystallizes in the tetragonal crystal system, with space group I41/a, and lattice parameters a = 576.2 pm and c = 1354.3 pm. In its structure, the Tc–O bond length is 172.3 pm, O–Tc–O bond angle is 108.34°.[2]
References
[edit]- ^ a b Klaus Schwochau (November 2008). Technetium: Chemistry and Radiopharmaceutical Applications. John Wiley & Sons. p. 129. ISBN 978-3527613373.
- ^ a b Jamie Weaver, Chuck Z. Soderquist, Nancy M. Washton, Andrew S. Lipton, Paul L. Gassman, Wayne W. Lukens, Albert A. Kruger, Nathalie A. Wall, John S. McCloy (February 2017). "Chemical Trends in Solid Alkali Pertechnetates". Inorganic Chemistry. 56 (5). Inorg. Chem.: 2533–2544. doi:10.1021/acs.inorgchem.6b02694. PMID 28221786.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
