Phosphorus sulfides
Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula P4Sn with n ≤ 10. Two are of commercial significance, phosphorus pentasulfide (P4S10), which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide (P4S3), used in the production of "strike anywhere matches".
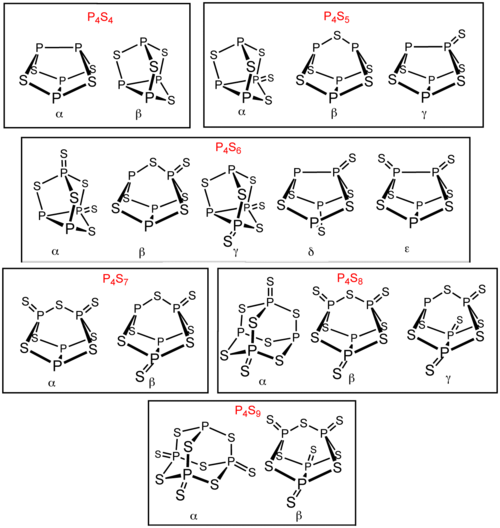
There are several other phosphorus sulfides in addition to P4S3 and P4S10. Six of these phosphorus sulfides exist as isomers: P4S4, P4S5, P4S6, P4S7, P4S8, and P4S9. These isomers are distinguished by Greek letter prefixes. The prefix is based on the order of the discovery of the isomers, not their structure.[1] All known molecular phosphorus sulfides contain a tetrahedral array of four phosphorus atoms.[2] P4S2 is also known but is unstable above −30 °C.[3]
Phosphorus monosulfide monomer, PS, is highly unstable and only exists at elevated temperatures. Its bond, worth about 55 kcal/mol, is about 2.4 angstroms long.[4]
Preparation
[edit]The main method for preparing these compounds is thermolysis of mixtures of phosphorus and sulfur. The product distributions can be analyzed by 31P-NMR spectroscopy. More selective syntheses entail:
- desulfurization, e.g. using triphenylphosphine and, complementarily,
- sulfidation using triphenylarsine sulfide.[5][6]
P4S3
[edit]Phosphorus sesquisulfide is prepared by treating red phosphorus with sulfur above 450 K,[7] followed by careful recrystallization with carbon disulfide and benzene. An alternative method involves the controlled fusion of white phosphorus with sulfur in an inert, non-flammable solvent.[8]
P4S4
[edit]The α- and β- forms of P4S4 can be prepared by treating the corresponding isomers of P4S3I2 with ((CH3)3Sn)2S:[7]
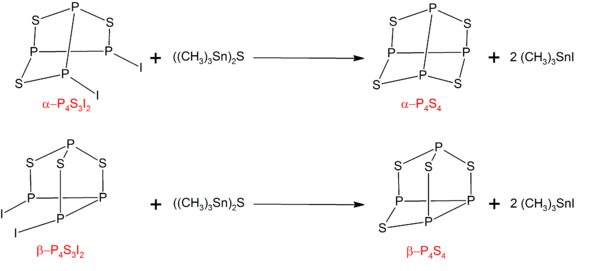
P4S3I2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.
P4S5
[edit]P4S5 can be prepared by treating stoichiometric amounts of P4S3 with sulfur in carbon disulfide solution, in the presence of light and a catalytic amount of iodine.[9] The respective product distribution is then analyzed by using 31P-NMR spectroscopy.
In particular, α-P4S5 can be easily made by the photochemical reaction of P4S10 with red phosphorus.[7] Note that P4S5 is unstable when heated, tending to disproportionate to P4S3 and P4S7 before reaching its melting point.[10]
P4S6
[edit]P4S6 can be made by abstracting a sulfur atom from P4S7 using triphenylphosphine:[7]
Treating α-P4S5 with Ph3AsS in CS2 also yields α-P4S6.[5] The two new polymorphs δ-P4S6 and ε-P4S6 can be made by treating α-P4S4 with Ph3SbS in CS2.[11]
P4S7
[edit]P4S7 is most conveniently made by direct union of the corresponding elements, and is one of the most easily purified binary phosphorus sulfides.[12]
- P4 + 7 S → P4S7
P4S8
[edit]β-P4S8 can be made by treating α-P4S7 with Ph3AsS in CS2, which yields a mixture between α-P4S7 and β-P4S8.[5]
P4S9
[edit]P4S9 can be made by two methods. One method involves the heating of P4S3 in excess sulfur.[7] Another method involves the heating of P4S7 and P4S10 in 1:2 mole ratio, where P4S9 is reversibly formed:[11]
- P4S7 + 2 P4S10 ⇌ 3 P4S9
P4S10
[edit]P4S10 is one of the most stable phosphorus sulfides. It is most easily made by heating white phosphorus with sulfur above 570 K in an evacuated tube.[13]
- P4 + 10 S → P4S10
See also
[edit]- Diphosphorus trisulfide (P2S3)
References
[edit]- ^ Jason, M. E.; Ngo, T.; Rahman, S. (1997). "Products and Mechanisms in the Oxidation of Phosphorus by Sulfur at Low Temperature". Inorg. Chem. 36 (12): 2633–2640. doi:10.1021/ic9614879.
- ^ Holleman, A. F.; Wiberg, E. Inorganic Chemistry. Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Heal, H. G. The Inorganic Heterocyclic Chemistry of Sulfur, Nitrogen, and Phosphorus Academic Press: London; 1980 ISBN 0-12-335680-6.
- ^ Almasi, Lucreţia (1971). "The Sulfur–Phosphorus Bond". In Senning, Alexander (ed.). Sulfur in Organic and Inorganic Chemistry. Vol. 1. New York: Marcel Dekker. p. 43. ISBN 0-8247-1615-9. LCCN 70-154612.
- ^ a b c Jason, M. E. (1997). "Transfer of Sulfur from Arsenic and Antimony Sulfides to Phosphorus Sulfides. Rational Syntheses of Several Less-Common P4Sn Species". Inorg. Chem. 36 (12): 2641–2646. doi:10.1021/ic9614881.
- ^ Nowottnick, H.; Blachnik, R. (1999). "Zwei neue Phosphorsulfide (Two New Phosphorus Sulfides)". Zeitschrift für anorganische und allgemeine Chemie. 625 (12): 1966–1968. doi:10.1002/(SICI)1521-3749(199912)625:12<1966::AID-ZAAC1966>3.0.CO;2-B.
- ^ a b c d e Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 484. ISBN 978-0-13-175553-6.
- ^ "Phosphorus trisulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 563.
- ^ "Phosphorus pentasulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 565.
- ^ A. Earnshaw; Norman Greenwood (2002). "Phosphorus". Chemistry of the elements, 2nd edition. Butterworth Heinemann. p. 508. ISBN 0750633654.
- ^ a b R. Bruce King (2005). "Phosphorus". Encyclopedia of Inorganic Chemistry, 2nd edition. Wiley. p. 3711. ISBN 9780470862100.
- ^ "Phosphorus heptasulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 566.
- ^ "Diphosphorus pentasulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 567.
