Radiolaria
| Radiolaria Temporal range:
| |
|---|---|

| |
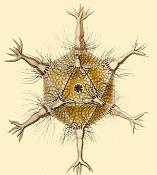
| Radiolaria illustration from the Challenger expedition 1873–76 | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Phylum: | Retaria |
| Subphylum: | Radiolaria Cavalier-Smith, 1987 |
| Classes | |
| |
| Part of a series on |
| Plankton |
|---|
 |
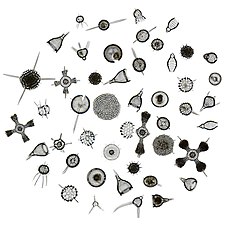
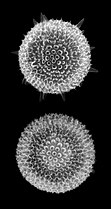
The Radiolaria, also called Radiozoa, are unicellular eukaryotes of diameter 0.1–0.2 mm that produce intricate mineral skeletons, typically with a central capsule dividing the cell into the inner and outer portions of endoplasm and ectoplasm. The elaborate mineral skeleton is usually made of silica.[1] They are found as zooplankton throughout the global ocean. As zooplankton, radiolarians are primarily heterotrophic, but many have photosynthetic endosymbionts and are, therefore, considered mixotrophs. The skeletal remains of some types of radiolarians make up a large part of the cover of the ocean floor as siliceous ooze. Due to their rapid change as species and intricate skeletons, radiolarians represent an important diagnostic fossil found from the Cambrian onwards.
Description
[edit]Radiolarians have many needle-like pseudopods supported by bundles of microtubules, which aid in the radiolarian's buoyancy. The cell nucleus and most other organelles are in the endoplasm, while the ectoplasm is filled with frothy vacuoles and lipid droplets, keeping them buoyant. The radiolarian can often contain symbiotic algae, especially zooxanthellae, which provide most of the cell's energy. Some of this organization is found among the heliozoa, but those lack central capsules and only produce simple scales and spines.
Some radiolarians are known for their resemblance to regular polyhedra, such as the icosahedron-shaped Circogonia icosahedra pictured below.
Taxonomy
[edit]The radiolarians belong to the supergroup Rhizaria together with (amoeboid or flagellate) Cercozoa and (shelled amoeboid) Foraminifera.[2] Traditionally the radiolarians have been divided into four groups—Acantharea, Nassellaria, Spumellaria and Phaeodarea. Phaeodaria is however now considered to be a Cercozoan.[3][4] Nassellaria and Spumellaria both produce siliceous skeletons and were therefore grouped together in the group Polycystina. Despite some initial suggestions to the contrary, this is also supported by molecular phylogenies. The Acantharea produce skeletons of strontium sulfate and is closely related to a peculiar genus, Sticholonche (Taxopodida), which lacks an internal skeleton and was for long time considered a heliozoan. The Radiolaria can therefore be divided into two major lineages: Polycystina (Spumellaria + Nassellaria) and Spasmaria (Acantharia + Taxopodida).[5][6]
There are several higher-order groups that have been detected in molecular analyses of environmental data. Particularly, groups related to Acantharia[7] and Spumellaria.[8] These groups are so far completely unknown in terms of morphology and physiology and the radiolarian diversity is therefore likely to be much higher than what is currently known.
The relationship between the Foraminifera and Radiolaria is also debated. Molecular trees support their close relationship—a grouping termed Retaria.[9] But whether they are sister lineages or whether the Foraminifera should be included within the Radiolaria is not known.
| Class | Order | Image | Families | Genera | Species | Description |
|---|---|---|---|---|---|---|
| Polycystinea | Nassellaria | 
|
... | |||
| Spumellaria | 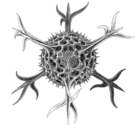
|
... | ||||
| Collodaria | 
|
... | ||||
| Acantharea | 
|
... | ||||
| Sticholonchea | Taxopodida | 
|
1 | 1 | 1 | ... |
Biogeography
[edit]
In the diagram on the right, a Illustrates generalized radiolarian provinces [10][11] and their relationship to water mass temperature (warm versus cool color shading) and circulation (gray arrows). Due to high-latitude water mass submergence under warm, stratified waters in lower latitudes, radiolarian species occupy habitats at multiple latitudes, and depths throughout the world oceans. Thus, marine sediments from the tropics reflect a composite of several vertically stacked faunal assemblages, some of which are contiguous with higher latitude surface assemblages. Sediments beneath polar waters include cosmopolitan deep-water radiolarians, as well as high-latitude endemic surface water species. Stars in (a) indicate the latitudes sampled, and the gray bars highlight the radiolarian assemblages included in each sedimentary composite. The horizontal purple bars indicate latitudes known for good radiolarian (silica) preservation, based on surface sediment composition.[12][13]
Data show that some species were extirpated from high latitudes but persisted in the tropics during the late Neogene, either by migration or range restriction (b). With predicted global warming, modern Southern Ocean species will not be able to use migration or range contraction to escape environmental stressors, because their preferred cold-water habitats are disappearing from the globe (c). However, tropical endemic species may expand their ranges toward midlatitudes. The color polygons in all three panels represent generalized radiolarian biogeographic provinces, as well as their relative water mass temperatures (cooler colors indicate cooler temperatures, and vice versa).[13]
-
Circogonia icosahedra, radiolarian species shaped like a regular icosahedron
-
Anthocyrtium hispidum Haeckel
Radiolarian shells
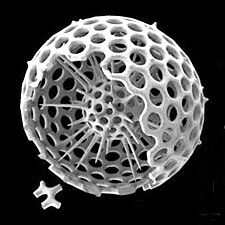
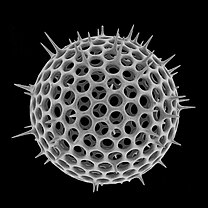
[edit]Radiolarians are unicellular predatory protists encased in elaborate globular shells (or "capsules"), usually made of silica and pierced with holes. Their name comes from the Latin for "radius". They catch prey by extending parts of their body through the holes. As with the silica frustules of diatoms, radiolarian shells can sink to the ocean floor when radiolarians die and become preserved as part of the ocean sediment. These remains, as microfossils, provide valuable information about past oceanic conditions.[14]
-
Like diatoms, radiolarians come in many shapes
-
Also like diatoms, radiolarian shells are usually made of silicate
-
However acantharian radiolarians have shells made from strontium sulfate crystals

-
Cutaway schematic diagram of a spherical radiolarian shell
-
Cladococcus abietinus
So I set to work on seeking a solution to the Morphogenesis Equations on a sphere. The theory was that a spherical organism was subject to diffusion across its surface membrane by an alien substance, eg sea-water. The Equations were:
The function , taken to be the radius vector from the centre to any point on the surface of the membrane, was argued to be representable as a series of normalised Legendre functions. The algebraic solution of the above equations ran to some 30 pages in my Thesis and are therefore not reproduced here. They are written in full in the book entitled “Morphogenesis” which is a tribute to Turing, edited by P. T. Saunders, published by North Holland, 1992.[16]
The algebraic solution of the equations revealed a family of solutions, corresponding to a parameter n, taking values 2, 4. 6.
When I had solved the algebraic equations, I then used the computer to plot the shape of the resulting organisms. Turing told me that there were real organisms corresponding to what I had produced. He said that they were described and depicted in the records of the voyages of HMS Challenger in the 19th Century.
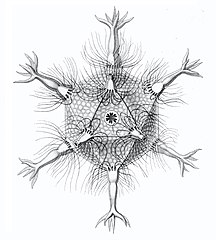
I solved the equations and produced a set of solutions which corresponded to the actual species of Radiolaria discovered by HMS Challenger in the 19th century. That expedition to the Pacific Ocean found eight variations in the growth patterns. These are shown in the following figures. The essential feature of the growth is the emergence of elongated "spines" protruding from the sphere at regular positions. Thus the species comprised two, six, twelve, and twenty, spine variations.
Bernard Richards, 2006 [17]
Diversity and morphogenesis
[edit]Bernard Richards, worked under the supervision of Alan Turing (1912–1954) at Manchester as one of Turing's last students, helping to validate Turing’s theory of morphogenesis.[18][19][20][21]
"Turing was keen to take forward the work that D’Arcy Thompson had published in On Growth and Form in 1917".[20]
closely replicate some radiolarian shell patterns[22]
| External videos | |
|---|---|
- Spine variations in radiolarians as discovered by HMS Challenger in the 19th century and drawn by Ernst Haeckel
-
Cromyatractus tetracelyphus with 2 spines
-
Circopus sexfurcus with 6 spines
-
Circopurus octahedrus with 6 spines and 8 faces
-
Circogonia icosahedra with 12 spines and 20 faces
-
Circorrhegma dodecahedra with 20 (incompletely drawn) spines and 12 faces
-
Cannocapsa stethoscopium with 20 spines
The gallery shows images of the radiolarians as extracted from drawings made by the German zoologist and polymath Ernst Haeckel in 1887.
- Turing, Alan (1952). "The Chemical Basis of Morphogenesis" (PDF). Philosophical Transactions of the Royal Society of London B. 237 (641): 37–72. Bibcode:1952RSPTB.237...37T. doi:10.1098/rstb.1952.0012. JSTOR 92463. S2CID 120437796.
- Richards, Bernard (2005-2006) "Turing, Richards and Morphogenesis", The Rutherford Journal, Volume 1.
Fossil record
[edit]The earliest known radiolaria date to the very start of the Cambrian period, appearing in the same beds as the first small shelly fauna—they may even be terminal Precambrian in age.[24][25][26][27] They have significant differences from later radiolaria, with a different silica lattice structure and few, if any, spikes on the test.[26] About ninety percent of known radiolarian species are extinct. The skeletons, or tests, of ancient radiolarians are used in geological dating, including for oil exploration and determination of ancient climates.[28]
Some common radiolarian fossils include Actinomma, Heliosphaera and Hexadoridium.
See also
[edit]References
[edit]- ^ Smalley, I.J. (1963). "Radiolarians:construction of spherical skeleton". Science. 140 (3565): 396–397. Bibcode:1963Sci...140..396S. doi:10.1126/science.140.3565.396. PMID 17815802. S2CID 28616246.
- ^ Pawlowski J, Burki F (2009). "Untangling the phylogeny of amoeboid protists". J. Eukaryot. Microbiol. 56 (1): 16–25. doi:10.1111/j.1550-7408.2008.00379.x. PMID 19335771.
- ^ Yuasa T, Takahashi O, Honda D, Mayama S (2005). "Phylogenetic analyses of the polycystine Radiolaria based on the 18s rDNA sequences of the Spumellarida and the Nassellarida". European Journal of Protistology. 41 (4): 287–298. doi:10.1016/j.ejop.2005.06.001.
- ^ Nikolaev SI, Berney C, Fahrni JF, et al. (May 2004). "The twilight of Heliozoa and rise of Rhizaria, an emerging supergroup of amoeboid eukaryotes". Proc. Natl. Acad. Sci. U.S.A. 101 (21): 8066–71. doi:10.1073/pnas.0308602101. PMC 419558. PMID 15148395.
- ^ Krabberød AK, Bråte J, Dolven JK, et al. (2011). "Radiolaria divided into Polycystina and Spasmaria in combined 18S and 28S rDNA phylogeny". PLOS ONE. 6 (8): e23526. Bibcode:2011PLoSO...623526K. doi:10.1371/journal.pone.0023526. PMC 3154480. PMID 21853146.
- ^ Cavalier-Smith T (December 1993). "Kingdom protozoa and its 18 phyla". Microbiol. Rev. 57 (4): 953–94. doi:10.1128/mmbr.57.4.953-994.1993. PMC 372943. PMID 8302218.
- ^ Decelle J, Suzuki N, Mahé F, de Vargas C, Not F (May 2012). "Molecular phylogeny and morphological evolution of the Acantharia (Radiolaria)". Protist. 163 (3): 435–50. doi:10.1016/j.protis.2011.10.002. PMID 22154393.
- ^ Not F, Gausling R, Azam F, Heidelberg JF, Worden AZ (May 2007). "Vertical distribution of picoeukaryotic diversity in the Sargasso Sea". Environ. Microbiol. 9 (5): 1233–52. Bibcode:2007EnvMi...9.1233N. doi:10.1111/j.1462-2920.2007.01247.x. PMID 17472637.
- ^ Cavalier-Smith T (July 1999). "Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree". J. Eukaryot. Microbiol. 46 (4): 347–66. doi:10.1111/j.1550-7408.1999.tb04614.x. PMID 18092388. S2CID 22759799.
- ^ Boltovskoy, D., Kling, S. A., Takahashi, K. & BjØrklund, K. (2010) "World atlas of distribution of recent Polycystina (Radiolaria)". Palaeontologia Electronica, 13: 1–230.
- ^ Casey, R. E., Spaw, J. M., & Kunze, F. R. (1982) "Polycystine radiolarian distribution and enhancements related to oceanographic conditions in a hypothetical ocean". Am. Assoc. Pet. Geol. Bull., 66: 319–332.
- ^ Lazarus, David B. (2011). "The deep-sea microfossil record of macroevolutionary change in plankton and its study". Geological Society, London, Special Publications. 358 (1): 141–166. Bibcode:2011GSLSP.358..141L. doi:10.1144/SP358.10. S2CID 128826639.
- ^ a b Trubovitz, Sarah; Lazarus, David; Renaudie, Johan; Noble, Paula J. (2020). "Marine plankton show threshold extinction response to Neogene climate change". Nature Communications. 11 (1): 5069. Bibcode:2020NatCo..11.5069T. doi:10.1038/s41467-020-18879-7. PMC 7582175. PMID 33093493.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Wassilieff, Maggy (2006) "Plankton - Animal plankton", Te Ara - the Encyclopedia of New Zealand. Accessed: 2 November 2019.
- ^ Kachovich, Sarah (2018) "Minds over Methods: Linking microfossils to tectonics" Blog of the Tectonics and Structural Geology Division of the European Geosciences Union.
- ^ Turing, Alan; Saunders, P. T. (1992). Morphogenesis (in Esperanto). Amsterdam: North-Holland. ISBN 978-0-08-093405-1. OCLC 680063781.
- ^ Richards, Bernard (2006) "Turing, Richards and Morphogenesis", The Rutherford Journal, Volume 1.
- ^ Richards, Bernard (1954), "The Morphogenesis of Radiolaria", MSc thesis, Manchester, UK: The University of Manchester
- ^ Richards, Bernard (2005). "Turing, Richards and morphogenesis". The Rutherford Journal. 1.
- ^ a b Richards, Bernard (2017). "Chapter 35 – Radiolaria: Validating the Turing theory". In Copeland, Jack; et al. (eds.). The Turing Guide. pp. 383–388.
- ^ Copeland, Jack; Bowen, Jonathan; Sprevak, Mark; Wilson, Robin; et al. (2017). "Notes on Contributors". The Turing Guide. Oxford University Press. p. 478. ISBN 978-0198747833.
- ^ Varea, C.; Aragon, J.L.; Barrio, R.A. (1999). "Turing patterns on a sphere". Physical Review E. 60 (4): 4588–92. Bibcode:1999PhRvE..60.4588V. doi:10.1103/PhysRevE.60.4588. PMID 11970318.
- ^ Kachovich, S., Sheng, J. and Aitchison, J.C., 2019. Adding a new dimension to investigations of early radiolarian evolution. Scientific reports, 9(1), pp.1-10. doi:10.1038/s41598-019-42771-0.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Chang, Shan; Feng, Qinglai; Zhang, Lei (14 August 2018). "New Siliceous Microfossils from the Terreneuvian Yanjiahe Formation, South China: The Possible Earliest Radiolarian Fossil Record". Journal of Earth Science. 29 (4): 912–919. Bibcode:2018JEaSc..29..912C. doi:10.1007/s12583-017-0960-0. S2CID 134890245.
- ^ Zhang, Ke; Feng, Qing-Lai (September 2019). "Early Cambrian radiolarians and sponge spicules from the Niujiaohe Formation in South China". Palaeoworld. 28 (3): 234–242. doi:10.1016/j.palwor.2019.04.001. S2CID 146452469.
- ^ a b A. Braun; J. Chen; D. Waloszek; A. Maas (2007), "First Early Cambrian Radiolaria", in Vickers-Rich, Patricia; Komarower, Patricia (eds.), The Rise and Fall of the Ediacaran Biota, Special publications, vol. 286, London: Geological Society, pp. 143–149, doi:10.1144/SP286.10, ISBN 978-1-86239-233-5, OCLC 156823511
- ^ Maletz, Jörg (June 2017). "The identification of putative Lower Cambrian Radiolaria". Revue de Micropaléontologie. 60 (2): 233–240. Bibcode:2017RvMic..60..233M. doi:10.1016/j.revmic.2017.04.001.
- ^ Zuckerman, L.D., Fellers, T.J., Alvarado, O., and Davidson, M.W. "Radiolarians", Molecular Expressions, Florida State University, 4 February 2004.
- Zettler, Linda A.; Sogin, ML; Caron, DA (1997). "Phylogenetic relationships between the Acantharea and the Polycystinea: A molecular perspective on Haeckel's Radiolaria". Proc. Natl. Acad. Sci. U.S.A. 94 (21): 11411–6. Bibcode:1997PNAS...9411411A. doi:10.1073/pnas.94.21.11411. PMC 23483. PMID 9326623.
- López-García P, Rodríguez-Valera F, Moreira D (January 2002). "Toward the monophyly of Haeckel's radiolaria: 18S rRNA environmental data support the sisterhood of polycystinea and acantharea". Mol. Biol. Evol. 19 (1): 118–121. doi:10.1093/oxfordjournals.molbev.a003976. PMID 11752197.
- Adl SM, Simpson AG, Farmer MA, et al. (2005). "The New Higher Level Classification of Eukaryotes with Emphasis on the Taxonomy of Protists". J. Eukaryot. Microbiol. 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873.
- Haeckel, Ernst (2005). Art Forms from the Ocean: The Radiolarian Atlas of 1862. Munich; London: Prestel Verlag. ISBN 978-3-7913-3327-4.
External links
[edit]- [1]Radiolarians
- Brodie, C. (February 2005). "Geometry and Pattern in Nature 3: The holes in radiolarian and diatom tests". Micscape (112). ISSN 1365-070X.
- Radiolaria.org
- Haeckel, Ernst (1862). Die Radiolarien (Rhizopoda radiaria). Berlin. Archived from the original on 2009-06-19. Retrieved 2007-09-07.
{{cite book}}: CS1 maint: location missing publisher (link) - Radiolaria—Droplet
- Tree Of Life—Radiolaria
- ^ Boltovskoy, Demetrio; Anderson, O. Roger; Correa, Nancy M. (2016). "Radiolaria and Phaeodaria". In Archibald, John M.; Simpson, Alastair G. B.; Slamovits, Claudio H.; Margulis, Lynn; Melkonian, Michael; Chapman, David J.; Corliss, John O. (eds.). Handbook of the Protists. Springer International Publishing. pp. 1–33. doi:10.1007/978-3-319-32669-6_19-1. ISBN 978-3-319-32669-6.