Nitrogen narcosis
| Inert gas narcosis [Nitrogen narcosis] | |
|---|---|
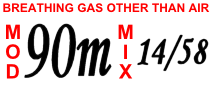 | |
| Divers breathe a mixture of oxygen, helium and nitrogen for deep dives to avoid the effects of narcosis. A cylinder label shows the maximum operating depth and mixture (oxygen/helium). | |
| Specialty | Medical toxicology |
Narcosis while diving (also known as nitrogen narcosis, inert gas narcosis, raptures of the deep, Martini effect) is a reversible alteration in consciousness that occurs while diving at depth. It is caused by the anesthetic effect of certain gases at high partial pressure. The Greek word νάρκωσις (narkōsis), "the act of making numb", is derived from νάρκη (narkē), "numbness, torpor", a term used by Homer and Hippocrates.[1] Narcosis produces a state similar to drunkenness (alcohol intoxication), or nitrous oxide inhalation. It can occur during shallow dives, but does not usually become noticeable at depths less than 30 metres (98 ft).
Except for helium and probably neon, all gases that can be breathed have a narcotic effect, although widely varying in degree.[2][3] The effect is consistently greater for gases with a higher lipid solubility, and although the mechanism of this phenomenon is still not fully clear, there is good evidence that the two properties are mechanistically related.[2] As depth increases, the mental impairment may become hazardous. Divers can learn to cope with some of the effects of narcosis, but it is impossible to develop a tolerance. Narcosis can affect all ambient pressure divers, although susceptibility varies widely among individuals and from dive to dive. The main modes of underwater diving that deal with its prevention and management are scuba diving and surface-supplied diving at depths greater than 30 metres (98 ft).
Narcosis may be completely reversed in a few minutes by ascending to a shallower depth, with no long-term effects. Thus narcosis while diving in open water rarely develops into a serious problem as long as the divers are aware of its symptoms, and are able to ascend to manage it. Diving much beyond 40 m (130 ft) is generally considered outside the scope of recreational diving. To dive at greater depths, as narcosis and oxygen toxicity become critical risk factors, gas mixtures such as trimix or heliox are used. These mixtures prevent or reduce narcosis by replacing some or all of the inert fraction of the breathing gas with non-narcotic helium. There is a synergy between carbon dioxide toxicity, and inert gas narcosis which is recognised but not fully understood. Conditions where high work of breathing due to gas density occur tend to exacerbate this effect.[4]
Classification
[edit]Narcosis results from breathing gases under elevated pressure, and may be classified by the principal gas involved. The noble gases, except helium and probably neon,[2] as well as nitrogen, oxygen and hydrogen cause a decrement in mental function, but their effect on psychomotor function (processes affecting the coordination of sensory or cognitive processes and motor activity) varies widely. The effect of carbon dioxide is a consistent diminution of mental and psychomotor function.[5] The noble gases argon, krypton, and xenon are more narcotic than nitrogen at a given pressure, and xenon has so much anesthetic activity that it is a usable anesthetic at 80% concentration and normal atmospheric pressure. Xenon has historically been too expensive to be used very much in practice, but it has been successfully used for surgical operations, and xenon anesthesia systems are still being proposed and designed.[6]
Signs and symptoms
[edit]
Due to its perception-altering effects, the onset of narcosis may be hard to recognize.[7][8] At its most benign, narcosis results in relief of anxiety – a feeling of tranquillity and mastery of the environment. These effects are essentially identical to various concentrations of nitrous oxide. They also resemble (though not as closely) the effects of alcohol and the familiar benzodiazepine drugs such as diazepam and alprazolam.[9] Such effects are not harmful unless they cause some immediate danger to go unrecognized and unaddressed. Once stabilized, the effects generally remain the same at a given depth, only worsening if the diver ventures deeper.[10]
The most dangerous aspects of narcosis are the impairment of judgement, multi-tasking and coordination, and the loss of decision-making ability and focus. Other effects include vertigo and visual or auditory disturbances. The syndrome may cause exhilaration, giddiness, extreme anxiety, depression, or paranoia, depending on the individual diver and the diver's medical or personal history. When more serious, the diver may feel overconfident, disregarding normal safe diving practices.[11] Slowed mental activity, as indicated by increased reaction time and increased errors in cognitive function, are effects which increase the risk of a diver mismanaging an incident.[12] Narcosis reduces both the perception of cold discomfort and shivering and thereby affects the production of body heat and consequently allows a faster drop in the core temperature in cold water, with reduced awareness of the developing problem.[12][13][14]
The relation of depth to narcosis is sometimes informally known as "Martini's law", the idea that narcosis results in the feeling of one martini for every 10 m (33 ft) below 20 m (66 ft) depth. This is a rough guide to give new divers a comparison with a situation they may be more familiar with.[15]
Reported signs and symptoms are summarized against typical depths in meters and feet of sea water in the following table, closely adapted from Deeper into Diving by Lippman and Mitchell:[11]
| Pressure (bar) | Depth (m) | Depth (ft) | Comments |
|---|---|---|---|
| 1–2 | 0–10 | 0–33 |
|
| 2–4 | 10–30 | 33–100 |
|
| 4–6 | 30–50 | 100–165 |
|
| 6–8 | 50–70 | 165–230 |
|
| 8–10 | 70–90 | 230–300 |
|
| 10+ | 90+ | 300+ |
|
Causes
[edit]| Some components of breathing gases and their relative narcotic potencies:[2][FN 1][3] | |
| Gas | Relative narcotic potency |
|---|---|
| He | 0.045 |
| Ne | 0.3 |
| H2 | 0.6 |
| N2 | 1.0 |
| O2 | 1.7 |
| Ar | 2.3 |
| Kr | 7.1 |
| CO2 | 20.0 |
| Xe | 25.6 |
The cause of narcosis is related to the increased solubility of gases in body tissues, as a result of the elevated pressures at depth (Henry's law).[17] It has been suggested that inert gases dissolving in the lipid bilayer of cell membranes cause narcosis.[18] More recently, researchers have been looking at neurotransmitter receptor protein mechanisms as a possible cause of narcosis.[19] The breathing gas mix entering the diver's lungs will have the same pressure as the surrounding water, known as the ambient pressure. After a change of depth, the partial pressure of inert gases in the blood passing through the brain catches up with ambient pressure within a minute or two, which results in a delayed change in narcotic effect after descending to a new depth.[7][20] Rapid compression potentiates narcosis owing to carbon dioxide retention.[21][22]
A divers' cognition may be affected on dives as shallow as 10 m (33 ft), but the changes are not usually noticeable.[23] There is no reliable method to predict the depth at which narcosis becomes noticeable, or the severity of the effect on an individual diver, as it may vary from dive to dive even on the same day.[7][22]
Significant impairment due to narcosis is an increasing risk below depths of about 30 m (100 ft), corresponding to an ambient pressure of about 4 bar (400 kPa).[7] Most sport scuba training organizations recommend depths of no more than 40 m (130 ft) because of the risk of narcosis.[15] When breathing air at depths of 90 m (300 ft) – an ambient pressure of about 10 bar (1,000 kPa) – narcosis in most divers leads to hallucinations, loss of memory, and unconsciousness.[21][24] A number of divers have died in attempts to set air depth records below 120 m (400 ft). Because of these incidents, Guinness World Records no longer reports on this figure.[25]
Narcosis has been compared with altitude sickness regarding its variability of onset (though not its symptoms); its effects depend on many factors, with variations between individuals. Thermal cold, stress, heavy work, fatigue, and carbon dioxide retention all increase the risk and severity of narcosis.[5][7] Carbon dioxide has a high narcotic potential and also causes increased blood flow to the brain, increasing the effects of other gases.[26] Increased risk of narcosis results from increasing the amount of carbon dioxide retained through heavy exercise, shallow or skip breathing, high work of breathing, or because of poor gas exchange in the lungs.[27][4]
Narcosis is known to be additive to even minimal alcohol intoxication.[28][29] Other sedative and analgesic drugs, such as opiate narcotics and benzodiazepines, add to narcosis.[28]
Mechanism
[edit]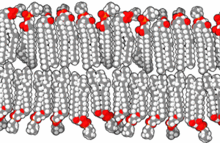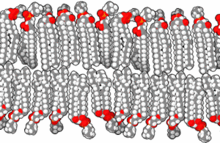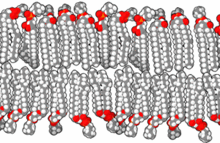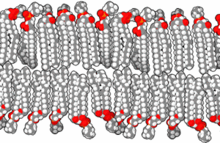
The precise mechanism is not well understood, but it appears to be the direct effect of gas dissolving into nerve membranes and causing temporary disruption in nerve transmissions. While the effect was first observed with air, other gases including argon, krypton and hydrogen cause very similar effects at higher than atmospheric pressure.[30] Some of these effects may be due to antagonism at NMDA receptors and potentiation of GABAA receptors,[31] similar to the mechanism of nonpolar anesthetics such diethyl ether or ethylene.[32] However, their reproduction by the very chemically inactive gas argon makes them unlikely to be a strictly chemical bonding to receptors in the usual sense of a chemical bond. An indirect physical effect – such as a change in membrane volume – would therefore be needed to affect the ligand-gated ion channels of nerve cells.[33] Trudell et al. have suggested non-chemical binding due to the attractive van der Waals force between proteins and inert gases.[34]
Similar to the mechanism of ethanol's effect, the increase of gas dissolved in nerve cell membranes may cause altered ion permeability properties of the neural cells' lipid bilayers. The partial pressure of a gas required to cause a measured degree of impairment correlates well with the lipid solubility of the gas: the greater the solubility, the less partial pressure is needed.[33]
An early theory, the Meyer-Overton hypothesis, suggested that narcosis happens when the gas penetrates the lipids of the brain's nerve cells, causing direct mechanical interference with the transmission of signals from one nerve cell to another.[17][18][22] More recently, specific types of chemically gated receptors in nerve cells have been identified as being involved with anesthesia and narcosis. However, the basic and most general underlying idea, that nerve transmission is altered in many diffuse areas of the brain as a result of gas molecules dissolved in the nerve cells' fatty membranes, remains largely unchallenged.[19][35]
Diagnosis and management
[edit]The symptoms of narcosis may be caused by other factors during a dive: ear problems causing disorientation or nausea;[36] early signs of oxygen toxicity causing visual disturbances;[37] carbon dioxide toxicity caused by rebreather scrubber malfunction, excessive work of breathing, or inappropriate breathing pattern, or hypothermia causing rapid breathing and shivering.[38] Nevertheless, the presence of any of these symptoms can imply narcosis. Alleviation of the effects upon ascending to a shallower depth will confirm the diagnosis. Given the setting, other likely conditions do not produce reversible effects. In the event of misdiagnosis when another condition is causing the symptoms, the initial management – ascending to a shallower depth – is still beneficial in most cases, as it is also the appropriate response for most of the alternative causes for the symptoms.[8]
The management of inert gas narcosis is usually simply to ascend to shallower depths, where much of the effect disappears within minutes.[39] Divers carrying multiple gas mixtures will usually switch to a mixture with more helium before significant narcosis is noticeable during descent. In the event of complications or other conditions being present, ascending remains the correct initial response unless it would violate decompression obligations. Should problems persist, it may be necessary to abort the dive. The decompression schedule can and should still be followed unless other conditions require emergency assistance.[40]
Inert gas narcosis can follow a gas switch to a decompression gas with higher nitrogen fraction during ascent, which may be confused with symptoms of decompression sickness, in a rare example of a situation in which it is not advisable to ascend immediately. If this is suspected to be the problem, it is better to switch back to the less narcotic gas if practicable, and adjust the decompression schedule to suit. This problem can be aggravated by the possibility of inert gas counterdiffusion, which is most likely to affect the inner ear, and can usually be avoided by a better selection of gas mixtures and switching depths.[41]
Prevention
[edit]
The most straightforward way to avoid nitrogen narcosis is for a diver to limit the depth of dives. The other main preventive measure is properly informed selection/choice of which gas to use for the particular dive under consideration.
Since narcosis becomes more severe as depth increases, a diver keeping to shallower depths can avoid serious narcosis. Most recreational training agencies will only certify entry level divers to depths of 18 to 20 m (60 to 70 ft), and at these depths narcosis does not present a significant risk. Further training is normally required for certification up to 30 m (100 ft) on air, and this training should include a discussion of narcosis, its effects, and management. Some diver training agencies offer specialized training to prepare recreational divers to go to depths of 40 m (130 ft), often consisting of further theory and some practice in deep dives under close supervision.[42][FN 2] Scuba organizations that train for diving beyond recreational depths,[FN 3] may exclude diving with gases that cause too much narcosis at depth in the average diver (such as the typical widely used nitrox mixtures used for most recreational diving), and strongly encourage the use of other breathing gas mixes containing helium in place of some or all of the nitrogen in air – such as trimix and heliox – because helium has no narcotic effect.[2][43] The use of these gases is considered to be technical diving and requires further training and certification.[15]
While the individual diver cannot predict exactly at what depth the onset of narcosis will occur on a given day, the first symptoms of narcosis for any given diver are often more predictable and personal. For example, one diver may have trouble with eye focus (close accommodation for middle-aged divers), another may experience feelings of euphoria, and another feelings of claustrophobia. Some divers report that they have hearing changes, and that the sound their exhaled bubbles make becomes different. Specialist training may help divers to identify these personal onset signs, which may then be used as a signal to ascend to avoid the narcosis, although severe narcosis may interfere with the judgement necessary to take preventive action.[39]
Deep dives should be made only after a gradual work-up to test the individual diver's sensitivity to increasing depths, taking note of reactions. Scientific evidence does not show that a diver can develop a resistance to the effects of narcosis at a given depth or become tolerant of it.[44]
Equivalent narcotic depth (END) is a commonly used way of expressing the narcotic effect of different breathing gases.[45] The National Oceanic and Atmospheric Administration (NOAA) Diving Manual now states that oxygen and nitrogen should be considered equally narcotic.[46] Standard tables, based on relative lipid solubilities, list conversion factors for narcotic effect of other gases.[47] For example, hydrogen at a given pressure has a narcotic effect equivalent to nitrogen at 0.55 times that pressure, so in principle it should be usable at more than twice the depth. Argon, however, has 2.33 times the narcotic effect of nitrogen, and is a poor choice as a breathing gas for diving (it is used as a drysuit inflation gas, owing to its low thermal conductivity). Some gases have other dangerous effects when breathed at pressure; for example, high-pressure oxygen can lead to oxygen toxicity. Although helium is the least intoxicating of the breathing gases, at greater depths it can cause high pressure nervous syndrome, a still mysterious but apparently unrelated phenomenon.[48] Inert gas narcosis is only one factor influencing the choice of gas mixture; the risks of decompression sickness and oxygen toxicity, work of breathing, cost, and other factors are also important.[49]
Because of similar and additive effects, divers should avoid sedating medications and drugs, such as cannabis and alcohol before any dive. A hangover, combined with the reduced physical capacity that goes with it, makes nitrogen narcosis more likely.[28] Experts recommend total abstinence from alcohol for at least 12 hours before diving, and longer for other drugs.[50]
Prognosis and epidemiology
[edit]Narcosis is potentially one of the most dangerous conditions to affect the scuba diver below about 30 m (100 ft). Except for occasional amnesia of events at depth, the effects of narcosis are entirely removed on ascent and therefore pose no problem in themselves, even for repeated, chronic or acute exposure.[7][22] Nevertheless, the severity of narcosis is unpredictable and it can be fatal while diving, as the result of inappropriate behavior in a dangerous environment.[22]
Tests have shown that all divers are affected by nitrogen narcosis, though some experience lesser effects than others. Even though it is possible that some divers can manage better than others because of learning to cope with the subjective impairment, the underlying behavioral effects remain.[32][51][52] These effects are particularly dangerous because a diver may feel they are not experiencing narcosis, yet still be affected by it.[7]
History
[edit]
French researcher Victor T. Junod was the first to describe symptoms of narcosis in 1834, noting "the functions of the brain are activated, imagination is lively, thoughts have a peculiar charm and, in some persons, symptoms of intoxication are present."[53][54] Junod suggested that narcosis resulted from pressure causing increased blood flow and hence stimulating nerve centers.[55] Walter Moxon (1836–1886), a prominent Victorian physician, hypothesized in 1881 that pressure forced blood to inaccessible parts of the body and the stagnant blood then resulted in emotional changes.[56] The first report of anesthetic potency being related to lipid solubility was published by Hans H. Meyer in 1899, entitled Zur Theorie der Alkoholnarkose. Two years later a similar theory was published independently by Charles Ernest Overton.[57] What became known as the Meyer-Overton hypothesis may be illustrated by a graph comparing narcotic potency with solubility in oil.
In 1939, Albert R. Behnke and O. D. Yarborough demonstrated that gases other than nitrogen also could cause narcosis.[58] For an inert gas the narcotic potency was found to be proportional to its lipid solubility. As hydrogen has only 0.55 the solubility of nitrogen, deep diving experiments using hydrox were conducted by Arne Zetterström between 1943 and 1945.[59] Jacques-Yves Cousteau in 1953 famously described it as "l'ivresse des grandes profondeurs" or the "rapture of the deep".[60]
Further research into the possible mechanisms of narcosis by anesthetic action led to the "minimum alveolar concentration" concept in 1965. This measures the relative concentration of different gases required to prevent motor response in 50% of subjects in response to stimulus, and shows similar results for anesthetic potency as the measurements of lipid solubility.[61] The (NOAA) Diving Manual was revised to recommend treating oxygen as if it were as narcotic as nitrogen, following research by Christian J. Lambertsen et al. in 1977 and 1978,[62] but this hypothesis has been challenged by more recent work.[63][64][65]
A study on the effects of the environment on inert gas narcosis published by Lafère et al. in 2016 concluded that pressure and gas composition may be the only significant external factors influencing inert gas narcosis. It also found that the onset of narcosis follows a short period of raised alertness during descent, and some of the effects persist for at least 30 minutes after the dive.[66][67] As of about 2020, research using critical flicker fusion frequency (CFFF) and EEG functional connectivity has shown sensitivity to nitrogen narcosis, but is not sensitive to helium partial pressure, in laboratory trials.[65][63][64]
See also
[edit]- Equivalent narcotic depth – Method for comparing the narcotic effects of a mixed diving gas with air
- Hydrogen narcosis – Psychotropic state induced by breathing hydrogen at high partial pressures
- Hypercapnia – Abnormally high tissue carbon dioxide levels
- Oxygen toxicity – Toxic effects of breathing oxygen at high partial pressures
- Substance-induced psychosis – Mental condition attributed to substance intoxication
Footnotes
[edit]- ^ Value for Krypton from 4th Edition, p. 176.
- ^ A number of technical diving agencies, such as TDI and IANTD teach "extended range" or "deep air" courses which teach diving to depths of up to 55 m (180 ft) without helium.
- ^ BSAC, SAA and other European training agencies teach recreational diving to a depth limit of 50 m (160 ft).
References
[edit]Notes
[edit]- ^ Askitopoulou, Helen; Ramoutsaki, Ioanna A; Konsolaki, Eleni (April 12, 2000). "Etymology and Literary History of Related Greek Words". Analgesia and Anesthesia. 91 (2). International Anesthesia Research Society: 486–491. Archived from the original on 2021-02-25. Retrieved 2010-06-09.
- ^ a b c d e Bennett & Rostain (2003), p. 305.
- ^ a b Bauer, Ralph W.; Way, Robert O. (1970). "Relative narcotic potencies of hydrogen, helium, nitrogen, and their mixtures". Archived from the original on 2016-07-01. Retrieved 2012-08-01.
- ^ a b Mitchell, Simon (20–22 April 2023). Developments in CO2 monitoring. Rebreather Forum 4. Valetta, Malta. Archived from the original on 16 April 2024. Retrieved 16 April 2024 – via GUE.
- ^ a b Hesser, C.M.; Fagraeus, L.; Adolfson, J. (1978). "Roles of nitrogen, oxygen, and carbon dioxide in compressed-air narcosis". Undersea and Hyperbaric Medicine. 5 (4). Undersea and Hyperbaric Medical Society, Inc: 391–400. ISSN 0093-5387. OCLC 2068005. PMID 734806. Archived from the original on July 31, 2009. Retrieved 2009-07-29.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Burov, N.E.; Kornienko, Liu; Makeev, G.N.; Potapov, V.N. (November–December 1999). "Clinical and experimental study of xenon anesthesia". Anesteziol Reanimatol (6): 56–60. PMID 11452771. Archived from the original on 2021-01-21. Retrieved 2008-11-03.
- ^ a b c d e f g Bennett & Rostain (2003), p. 301.
- ^ Hobbs M. (2008). "Subjective and behavioural responses to nitrogen narcosis and alcohol". Undersea & Hyperbaric Medicine. 35 (3): 175–84. PMID 18619113. Archived from the original on April 15, 2013. Retrieved 2009-08-07.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Lippmann & Mitchell (2005), p. 103.
- ^ a b Lippmann & Mitchell (2005), p. 105.
- ^ a b c Doolette, David J. (August 2008). "2: Inert Gas Narcosis". In Mount, Tom; Dituri, Joseph (eds.). Exploration and Mixed Gas Diving Encyclopedia (1st ed.). Miami Shores, Florida: International Association of Nitrox Divers. pp. 33–40. ISBN 978-0-915539-10-9.
- ^ Mekjavic, Igor B.; Passias, T.; Sundberg, Carl Johan; Eiken, O. (April 1994). "Perception of thermal comfort during narcosis". Undersea & Hyperbaric Medicine. 21 (1). Undersea and Hyperbaric Medical Society: 9–19. PMID 8180569. Archived from the original on 27 December 2016. Retrieved 26 December 2011.
- ^ Mekjavic, Igor B.; Savić, S.A.; Eiken, O. (June 1995). "Nitrogen narcosis attenuates shivering thermogenesis". Journal of Applied Physiology. 78 (6). American Physiological Society: 2241–4. doi:10.1152/jappl.1995.78.6.2241. PMID 7665424.
- ^ a b c Brylske, A. (2006). Encyclopedia of Recreational Diving (3rd ed.). United States: Professional Association of Diving Instructors. ISBN 1-878663-01-1.
- ^ "The Call of the Wah-Wah". InDepth. Retrieved 15 August 2024.
- ^ a b Bennett & Rostain (2003), p. 308.
- ^ a b Paton, William (1975). "Diver narcosis, from man to cell membrane". Journal of the South Pacific Underwater Medicine Society (First Published at Oceans 2000 Conference). 5 (2). Archived from the original on April 15, 2013. Retrieved 2008-12-23.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ a b Rostain, Jean C; Balon N (2006). "Recent neurochemical basis of inert gas narcosis and pressure effects". Undersea and Hyperbaric Medicine. 33 (3): 197–204. PMID 16869533. Archived from the original on August 20, 2008. Retrieved 2008-12-23.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Case, E.M.; Haldane, John Burdon Sanderson (1941). "Human physiology under high pressure". Journal of Hygiene. 41 (3): 225–49. doi:10.1017/S0022172400012432. PMC 2199778. PMID 20475589.
- ^ a b Bennett & Rostain (2003), p. 303.
- ^ a b c d e Hamilton, R.W.; Kizer, K.W., eds. (1985). "Nitrogen Narcosis". 29th Undersea and Hyperbaric Medical Society Workshop (UHMS Publication Number 64WS(NN)4-26-85). Bethesda, MD: Undersea and Hyperbaric Medical Society. Archived from the original on August 20, 2008. Retrieved 2008-12-23.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Petri, NM (2003). "Change in strategy of solving psychological tests: evidence of nitrogen narcosis in shallow air-diving". Undersea and Hyperbaric Medicine. 30 (4). Undersea and Hyperbaric Medical Society, Inc: 293–303. PMID 14756232. Archived from the original on December 31, 2007. Retrieved 2008-12-23.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Hill, Leonard; David, R.H.; Selby, R.P.; et al. (1933). "Deep diving and ordinary diving". Report of a Committee Appointed by the British Admiralty.
- ^ PSAI Philippines. "Professional Scuba Association International History". Professional Scuba Association International – Philippines. Archived from the original on 2009-01-01. Retrieved 2008-10-31.
- ^ Kety, Seymour S.; Schmidt, Carl F (1948). "The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men". Journal of Clinical Investigation. 27 (4): 484–492. doi:10.1172/JCI101995. ISSN 0021-9738. PMC 439519. PMID 16695569.
- ^ Lippmann & Mitchell (2005), pp. 110–3.
- ^ a b c Fowler, B.; Hamilton, K.; Porlier, G. (1986). "Effects of ethanol and amphetamine on inert gas narcosis in humans". Undersea Biomedical Research. 13 (3): 345–54. PMID 3775969. Archived from the original on October 7, 2008. Retrieved 2008-12-23.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Michalodimitrakis, E; Patsalis, A (1987). "Nitrogen narcosis and alcohol consumption—a scuba diving fatality". Journal of Forensic Sciences. 32 (4): 1095–7. doi:10.1520/JFS12421J. PMID 3612064.
- ^ Bennett & Rostain (2003), p. 304.
- ^ Hapfelmeier, Gerhard; Zieglgänsberger, Walter; Haseneder, Rainer; Schneck, Hajo; Kochs, Eberhard (December 2000). "Nitrous oxide and xenon increase the efficacy of GABA at recombinant mammalian GABA(A) receptors". Anesthesia and Analgesia. 91 (6): 1542–9. doi:10.1097/00000539-200012000-00045. PMID 11094015. S2CID 71906242. Archived from the original on 2008-12-01. Retrieved 2009-07-29.
- ^ a b Hamilton, K.; Laliberté, M.F.; Fowler, B. (1995). "Dissociation of the behavioral and subjective components of nitrogen narcosis and diver adaptation". Undersea & Hyperbaric Medicine. 22 (1): 41–49. ISSN 1066-2936. OCLC 26915585. PMID 7742709. Archived from the original on May 14, 2011. Retrieved 2009-07-29.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ a b Franks, N.P.; Lieb, W.R. (1994). "Molecular and cellular mechanisms of general anaesthesia". Nature. 367 (6464): 607–14. Bibcode:1994Natur.367..607F. doi:10.1038/367607a0. PMID 7509043. S2CID 4357493.
- ^ Trudell, J.R.; Koblin, D.D.; Eger, E.I. (1998). "A molecular description of how noble gases and nitrogen bind to a model site of anesthetic action". Anesthesia and Analgesia. 87 (2): 411–8. doi:10.1097/00000539-199808000-00034. PMID 9706942. S2CID 20293831. Archived from the original on 2006-09-13. Retrieved 2008-12-01.
- ^ Smith, EB (July 1987). "Priestley lecture 1986. On the science of deep-sea diving—observations on the respiration of different kinds of air". Undersea & Hyperbaric Medicine. 14 (4): 347–69. PMID 3307084. Archived from the original on January 29, 2009. Retrieved 2009-07-29.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Molvaer, Otto I (2003). "Otorhinolaryngological aspects of diving". In Brubakk, Alf O; Neuman, Tom S (eds.). Bennett & Rostain's physiology and medicine of diving (5th ed.). United States: Saunders Ltd. p. 234. ISBN 0-7020-2571-2. OCLC 51607923.
- ^ Clark, James M; Thom, Stephen R (2003). "Oxygen under pressure". In Brubakk, Alf O; Neuman, Tom S (eds.). Bennett & Rostain's physiology and medicine of diving (5th ed.). United States: Saunders Ltd. p. 374. ISBN 0-7020-2571-2. OCLC 51607923.
- ^ Mekjavic, Igor B; Tipton, Michael J; Eiken, Ola (2003). "Thermal considerations in diving". In Brubakk, Alf O; Neuman, Tom S (eds.). Bennett & Rostain's physiology and medicine of diving (5th ed.). United States: Saunders Ltd. p. 129. ISBN 0-7020-2571-2. OCLC 51607923.
- ^ a b Lippmann & Mitchell (2005), p. 106.
- ^ Program (U.S.), NOAA Diving (2001). NOAA Diving Manual: Diving for Science and Technology. National Oceanic and Atmospheric Administration, Office of Oceanic and Atmospheric Research, National Undersea Research Program, Office of Marine and Aviation Operations, NOAA Diving Program. ISBN 978-0-941332-70-5. Archived from the original on 2024-04-16. Retrieved 2023-05-05.
- ^ "Extended Range Diver". International Training. 2009. Archived from the original on 2013-09-12. Retrieved 2013-01-24.
- ^ Hamilton Jr, R.W.; Schreiner, H.R., eds. (1975). "Development of Decompression Procedures for Depths in Excess of 400 feet". 9th Undersea and Hyperbaric Medical Society Workshop (UHMS Publication Number WS2–28–76). Bethesda, MD: Undersea and Hyperbaric Medical Society: 272. Archived from the original on August 20, 2008. Retrieved 2008-12-23.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Hamilton, K.; Laliberté, M.F.; Heslegrave, R. (1992). "Subjective and behavioral effects associated with repeated exposure to narcosis". Aviation, Space, and Environmental Medicine. 63 (10): 865–9. PMID 1417647.
- ^ IANTD (January 1, 2009). "IANTD Scuba & CCR, PSCR & SCR Rebreather Diver Programs (Recreational Trimix Diver)". IANTD. Archived from the original on April 2, 2009. Retrieved 2009-03-22.
- ^ "Mixed-Gas & Oxygen". NOAA Diving Manual, Diving for Science and Technology. 4th. National Oceanic and Atmospheric Administration. 2002.
[16.3.1.2.4] ... since oxygen has some narcotic properties, it is appropriate to include the oxygen in the END calculation when using trimixes (Lambersten et al. 1977,1978). The non-helium portion (i.e., the sum of the oxygen and the nitrogen) is to be regarded as having the same narcotic potency as an equivalent partial pressure of nitrogen in air, regardless of the proportions of oxygen and nitrogen.
- ^ Anttila, Matti (2000). "Narcotic factors of gases". Archived from the original on 2013-12-09. Retrieved 2008-06-10.
- ^ Bennett, Peter; Rostain, Jean Claude (2003). "The High Pressure Nervous Syndrome". In Brubakk, Alf O.; Neuman, Tom S. (eds.). Bennett & Rostain's physiology and medicine of diving (5th ed.). United States: Saunders Ltd. pp. 323–57. ISBN 0-7020-2571-2. OCLC 51607923.
- ^ Lippmann & Mitchell (2005), pp. 430–1.
- ^ St Leger Dowse, Marguerite (2008). "Diving Officer's Conference presentations". British Sub-Aqua Club. Archived from the original on 2011-06-14. Retrieved 2009-08-16.
- ^ Fowler, B.; Ackles, K.N.; Porlier, G. (1985). "Effects of inert gas narcosis on behavior—a critical review". Undersea & Hyperbaric Medicine. 12 (4): 369–402. ISSN 0093-5387. OCLC 2068005. PMID 4082343. Archived from the original on 2010-12-25. Retrieved 2009-07-29.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Rogers, WH; Moeller, G (1989). "Effect of brief, repeated hyperbaric exposures on susceptibility to nitrogen narcosis". Undersea & Hyperbaric Medicine. 16 (3): 227–32. ISSN 0093-5387. OCLC 2068005. PMID 2741255. Archived from the original on 2009-09-01. Retrieved 2009-07-29.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Bennett & Rostain (2003), p. 300.
- ^ Junod, Victor T. (1834). "Recherches physiologiques et thérapeutiques sur les effets de la compression et de la raréfaction de l'air". Revue médicale française et étrangère: Journal des progrès de la médecine hippocratique. Chez Gabon et compagnie: 350–68. Retrieved 2009-06-04.
- ^ Bennett & Rostain (2003), p. 306.
- ^ Moxon, Walter (1881). "Croonian lectures on the influence of the circulation on the nervous system". British Medical Journal. 1 (1057): 491–7. doi:10.1136/bmj.1.1057.491. PMC 2263574. PMID 20749830.
Moxon, Walter (1881). "Croonian lectures on the influence of the circulation on the nervous system". British Medical Journal. 1 (1059): 583–5. doi:10.1136/bmj.1.1059.583. PMC 2263398. PMID 20749844. - ^ Overton, Charles Ernest (1901). "Studien Über Die Narkose". Allgemeiner Pharmakologie (in German). Institut für Pharmakologie.
- ^ Behnke, A.R.; Yarborough, O.D. (1939). "Respiratory resistance, oil-water solubility and mental effects of argon compared with helium and nitrogen". American Journal of Physiology. 126 (2): 409–15. doi:10.1152/ajplegacy.1939.126.2.409.
- ^ Ornhagen, H (1984). "Hydrogen-Oxygen (Hydrox) breathing at 1.3 MPa". FOA Rapport C58015-H1. Stockholm: National Defence Research Institute. ISSN 0347-7665.
- ^ Cousteau, Jacques-Yves; Dumas, Frédéric (1953). The Silent World: A Story of Undersea Discovery and Adventure. Harper & Brothers Publishers. p. 266. ISBN 0-7922-6796-6.
- ^ Eger, EI; Saidman, LJ; Brandstater, B (1965). "Minimum alveolar anesthetic concentration: a standard of anesthetic potency". Anesthesiology. 26 (6): 756–63. doi:10.1097/00000542-196511000-00010. PMID 5844267.
- ^ Lambertsen, Christian J.; Gelfand, R.; Clark, J.M. (1978). "University of Pennsylvania Institute for Environmental Medicine report, 1978". University of Pennsylvania. Institute for Environmental Medicine. Archived from the original on June 12, 2010. Retrieved 2009-03-22.
- ^ a b Vrijdag, Xavier (1 February 2023). "Is Oxygen Narcosis A Thing?". gue.com. Archived from the original on 30 March 2023. Retrieved 30 March 2023.
- ^ a b Vrijdag, Xavier C. E.; van Waart, Hanna; Sames, Chris; Mitchell, Simon J.; Sleigh, Jamie W. (20 July 2022). "Does hyperbaric oxygen cause narcosis or hyperexcitability? A quantitative EEG analysis". Physiological Reports. 10 (14): e15386. doi:10.14814/phy2.15386. PMC 9300958. PMID 35859332.
- ^ a b Vrijdag, X.C.; van Waart, H.; Sleigh, J.W.; Balestra, C.; Mitchell, S.J. (20 December 2020). "Investigating critical flicker fusion frequency for monitoring gas narcosis in divers". Diving Hyperb Med. 50 (4): 377–385. doi:10.28920/dhm50.4.377-385. PMC 7872789. PMID 33325019.
- ^ Menduno, Michael (14 May 2020). "Measuring Inert Gas Narcosis". alertdiver.eu. DAN Europe. Archived from the original on 30 March 2023. Retrieved 4 April 2023.
- ^ Lafere, P; Hemelryck, W; Germonpre, P; Matity, L; Guerrero, F; Balestra, C (2019-06-30). "Early detection of diving-related cognitive impairment of different nitrogen-oxygen gas mixtures using critical flicker fusion frequency". Diving and Hyperbaric Medicine. 49 (2): 119–126. doi:10.28920/dhm49.2.119-126. PMC 6704008. PMID 31177518.
Sources
[edit]- Bennett, Peter; Rostain, Jean Claude (2003). "Inert Gas Narcosis". In Brubakk, Alf O.; Neuman, Tom S (eds.). Bennett and Elliott's physiology and medicine of diving (5th ed.). United States: Saunders. ISBN 0-7020-2571-2. OCLC 51607923.
- Lippmann, John; Mitchell, Simon J. (2005). "Nitrogen narcosis". Deeper into Diving (2nd ed.). Victoria, Australia: J. L. Publications. pp. 103–8. ISBN 0-9752290-1-X. OCLC 66524750.
- U.S. Navy Supervisor of Diving (2008). U.S. Navy Diving Manual (PDF). SS521-AG-PRO-010, revision 6. U.S. Naval Sea Systems Command. Archived from the original (PDF) on 2014-12-10. Retrieved 2014-01-21.
External links
[edit]- Undersea and Hyperbaric Medical Society Scientific body, publications about nitrogen narcosis.
- Diving Diseases Research Centre (DDRC) UK charity dedicated to treatment of diving diseases.
- Campbell, Ernest S. (2009-06-25). "Diving While Using Marijuana". Retrieved 2009-08-25. ScubaDoc's overview of marijuana and diving.
- Campbell, Ernest S. (2009-05-03). "Alcohol and Diving". Archived from the original on 2007-04-30. Retrieved 2009-08-25. ScubaDoc's overview of alcohol and diving.
- Campbell, George D. (2009-02-01). "Nitrogen Narcosis". Diving with Deep-Six. Retrieved 2009-08-25.
