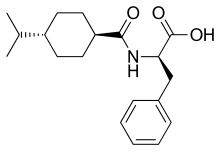
Nateglinide
 | |
| Clinical data | |
|---|---|
| Trade names | Starlix |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699057 |
| License data |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 98% |
| Elimination half-life | 1.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.170.086 |
| Chemical and physical data | |
| Formula | C19H27NO3 |
| Molar mass | 317.429 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nateglinide (INN, trade name Starlix) is a drug for the treatment of type 2 diabetes. Nateglinide was developed by Ajinomoto, a Japanese company and sold by the Swiss pharmaceutical company Novartis.
Nateglinide belongs to the meglitinide class of blood glucose-lowering drugs.
Pharmacology
[edit]Nateglinide lowers blood glucose by stimulating the release of insulin from the pancreas. It achieves this by closing ATP-dependent potassium channels in the membrane of the β cells. This depolarizes the β cells and causes voltage-gated calcium channels to open. The resulting calcium influx induces fusion of insulin-containing vesicles with the cell membrane, and insulin secretion occurs.
Contraindications
[edit]Nateglinide is contraindicated in patients who:
- have known hypersensitivity to the compound or any ingredient in the formulation.
- are affected with type 1 (namely insulin-dependent) diabetes mellitus.
- are in diabetic ketoacidosis.
Comparisons with other drugs for type 2 diabetes
[edit]A study funded by Novo Nordisk, the U.S. distributor for Repaglinide, compared their product with Nateglinide in "A randomized, parallel-group, open-label, multicenter 16-week clinical trial".[1] They concluded that the two were similar, but "repaglinide monotherapy was significantly more effective than nateglinide monotherapy in reducing HbA1c and FPG values after 16 weeks of therapy."
Dosage
[edit]Nateglinide is delivered in 60 mg & 120 mg tablet form.
See also
[edit]References
[edit]- ^ Rosenstock J, Hassman DR, Madder RD, Brazinsky SA, Farrell J, Khutoryansky N, Hale PM (June 2004). "Repaglinide versus nateglinide monotherapy: a randomized, multicenter study". Diabetes Care. 27 (6). American Diabetes Association: 1265–70. doi:10.2337/diacare.27.6.1265. PMID 15161773. Retrieved 2014-11-20.
External links
[edit]- Starlix - website of the manufacturer.
- How Nateglinide Works - website of the manufacturer.
