Mitomycin C
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mitosol, Mutamycin, Jelmyto |
| Other names | UGN-101 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682415 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous, topical |
| Drug class | Antineoplastic agent |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 8–48 min |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.008 |
| Chemical and physical data | |
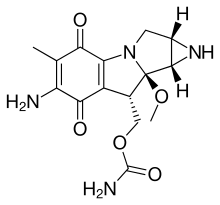
| Formula | C15H18N4O5 |
| Molar mass | 334.332 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 360 °C (680 °F) |
| Solubility in water | 8.43 g L−1 |
| |
| |
Mitomycin C is a mitomycin that is used as a chemotherapeutic agent by virtue of its antitumour activity.
Medical uses
[edit]It is given intravenously to treat upper gastro-intestinal cancers (e.g. esophageal carcinoma), anal cancers, and breast cancers, as well as by bladder instillation for superficial bladder tumours.
Mitomycin C has also been used topically rather than intravenously in several areas. The first is cancers, particularly bladder cancers and intraperitoneal tumours. It is now well known that a single instillation of this agent within 6 hours of bladder tumor resection can prevent recurrence. The second is in eye surgery where mitomycin C 0.02% is applied topically to prevent scarring during glaucoma filtering surgery and to prevent haze after PRK or LASIK; mitomycin C has also been shown to reduce fibrosis in strabismus surgery.[6] The third is in esophageal and tracheal stenosis where application of mitomycin C onto the mucosa immediately following dilatation will decrease re-stenosis by decreasing the production of fibroblasts and scar tissue.
In April 2020, mitomycin gel, sold under the brand name Jelmyto, was approved in the United States for the treatment of low-grade upper tract urothelial cancer (UTUC).[7][8][9] Urothelial cancer is a cancer of the lining of the urinary system.[7]
Mitomycin is also used as a chemotherapeutic agent in glaucoma surgery.
Contraindications
[edit]Pregnant women should not take mitomycin gel because it may cause harm to a developing fetus or newborn baby.[7]
Side effects
[edit]It causes delayed bone marrow toxicity and therefore it is usually administered at 6-weekly intervals. Prolonged use may result in permanent bone-marrow damage. It may also cause lung fibrosis and renal damage.
Anticancer treatments with chemotherapeutic agents often impair brain cell function leading to memory loss and cognitive dysfunction. In order to understand the basis of these impairments, mice were treated with mitomycin C, a chemotherapeutic agent, and cells of the prefrontal cortex were examined.[10] This treatment resulted in an increase of the oxidative DNA damage 8-oxo-dG, a decrease in the enzyme OGG1 that ordinarily repairs such damage and epigenetic alterations. These alterations at the DNA level may explain, at least in part, the impairments of cognitive function after chemotherapy.[11]
Common side effects are ureteric obstruction (narrowing or blockage of the ureter that may lead to excess fluid in the kidney due to a backup of urine), flank pain (pain occurring on the side of the body), urinary tract infection, hematuria (blood in the urine), renal dysfunction (inability of the kidney to function in its designed capacity), fatigue, nausea, abdominal pain, dysuria (painful or difficult urination) and vomiting.[7]
Pharmacology
[edit]Mitomycin C is a potent DNA crosslinker. A single crosslink per genome has shown to be effective in killing bacteria. This is accomplished by reductive activation of mitomycin to form a mitosene, which reacts successively via N-alkylation of two DNA bases. Both alkylations are sequence specific for a guanine nucleoside in the sequence 5'-CpG-3'.[12]
Mitomycin gel is an alkylating drug, meaning it inhibits the transcription of DNA into RNA, stopping protein synthesis and taking away the cancer cell's ability to multiply.[7]
History
[edit]Mitomycin was discovered in 1955 by Japanese scientists in cultures of the microorganism Streptomyces caespitosus.[12] Mitomycin C was isolated as purple crystals by Wakaki and his coworkers from Kyowa Hakko Kogyo in 1956.[13]
It was approved based on the results of the OLYMPUS (NCT02793128) multicenter trial involving 71 subjects with low-grade upper urinary tract urothelial cancer (UTUC).[7][8] These subjects had never undergone treatment (treatment-naïve) or had recurrent low-grade non-invasive UTUC with at least one measurable papillary tumor (a tumor shaped like a small mushroom with its stem attached to the inner lining of an organ) located above the ureteropelvic junction.[7][8] Subjects received mitomycin gel once a week (mitomycin gel 4 mg per mL instillations via ureteral catheter or nephrostomy tube) for six weeks and, if assessed as a complete response (complete disappearance of the papillary tumor), monthly for up to eleven additional months.[7][8] Efficacy of mitomycin gel was evaluated using urine cytology (a test to look for abnormal cells in a subjects's urine), ureteroscopy (an examination of the upper urinary tract) and biopsy (if warranted) three months following the initiation of therapy.[7]
The primary endpoint was complete response at three months following initiation of therapy.[7][8] A complete response was found in 41 of the 71 subjects (58%) following six treatments of mitomycin gel administered weekly.[7][8] Durability of the effect of mitomycin gel in subjects with a complete response was also evaluated using urine cytology, ureteroscopy and biopsy (if warranted) every three months for a year following the initiation of therapy.[7][8] Nineteen subjects (46%) who achieved a complete response continued to have a complete response at the twelve-month mark.[7][8]
The US Food and Drug Administration (FDA) granted the application for mitomycin gel priority review along with breakthrough therapy, fast track, and orphan drug designations.[7] The FDA granted approval of Jelmyto to UroGen Pharma, Inc.[7]
Research
[edit]Potential bis-alkylating heterocyclic quinones were synthesised in order to explore their antitumoral activities by bioreductive alkylation.[14]
In the bacterium Legionella pneumophila, mitomycin C induces competence, a condition necessary for the process of natural transformation that transfers DNA and promotes recombination between cells.[15] Exposure of the fruitfly Drosophila melanogaster to mitomycin C increases recombination during meiosis, a key stage of the sexual cycle.[16] It has been suggested that during sexual process in prokaryotes (transformation) and eukaryotes (meiosis) DNA cross-links and other damages introduced by mitomycin C may be removed by recombinational repair.[17]
References
[edit]- ^ Reiss GJ (2011). "KUWQIF: Mitomycin C Dihydrate, also known as (6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl)methyl carbamate dihydrate". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. doi:10.5517/ccdc.csd.cc12bt29. Archived from the original on 19 November 2021. Retrieved 3 November 2021.
- ^ "Mitomycin (Mutamycin) Use During Pregnancy". Drugs.com. 19 August 2019. Archived from the original on 22 October 2020. Retrieved 15 April 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Mitocin mitomycin 20 mg powder for injection vial (370360)". Therapeutic Goods Administration (TGA). 12 August 2022. Archived from the original on 18 March 2023. Retrieved 29 April 2023.
- ^ "Mitocin (Echo Therapeutics Pty Ltd)". Therapeutic Goods Administration (TGA). 28 September 2022. Archived from the original on 18 March 2023. Retrieved 29 April 2023.
- ^ Kersey JP, Vivian AJ (July–September 2008). "Mitomycin and amniotic membrane: a new method of reducing adhesions and fibrosis in strabismus surgery". Strabismus. 16 (3): 116–118. doi:10.1080/09273970802405493. PMID 18788060. S2CID 32321781.
- ^ a b c d e f g h i j k l m n o "FDA Approves First Therapy for Treatment of Low-Grade Upper Tract Urothelial Cancer". U.S. Food and Drug Administration (FDA) (Press release). 15 April 2020. Archived from the original on 15 April 2020. Retrieved 15 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f g h "FDA approves mitomycin for low-grade upper tract urothelial cancer". U.S. Food and Drug Administration (FDA). 15 April 2020. Archived from the original on 15 April 2020. Retrieved 15 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Jelmyto: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 15 April 2020. Retrieved 15 April 2020.
- ^ Kovalchuk A, Rodriguez-Juarez R, Ilnytskyy Y, Byeon B, Shpyleva S, Melnyk S, et al. (April 2016). "Sex-specific effects of cytotoxic chemotherapy agents cyclophosphamide and mitomycin C on gene expression, oxidative DNA damage, and epigenetic alterations in the prefrontal cortex and hippocampus - an aging connection". Aging. 8 (4): 697–711. doi:10.18632/aging.100920. PMC 4925823. PMID 27032448.
- ^ Kovalchuk A, Kolb B (July 2017). "Chemo brain: From discerning mechanisms to lifting the brain fog-An aging connection". Cell Cycle. 16 (14): 1345–1349. doi:10.1080/15384101.2017.1334022. PMC 5539816. PMID 28657421.
- ^ a b Tomasz M (September 1995). "Mitomycin C: small, fast and deadly (but very selective)". Chemistry & Biology. 2 (9): 575–579. doi:10.1016/1074-5521(95)90120-5. PMID 9383461.
- ^ Wakaki S, Marumo H, Tomioka K, Shimizu G, Kato E, Kamada H, et al. (May 1958). "Isolation of new fractions of antitumor mitomycins". Antibiotics & Chemotherapy. 8 (5): 228–240. PMID 24544727.
- ^ Renault J, Baron M, Mailliet P, Giorgirenault S, Paoletti C, Cros S (1981). "Heterocyclic quinones 2. Quinoxaline-5,6-(and 5-8)-diones - Potential antitumoral agents". Eur. J. Med. Chem. 16 (6): 545–550.
- ^ Charpentier X, Kay E, Schneider D, Shuman HA (March 2011). "Antibiotics and UV radiation induce competence for natural transformation in Legionella pneumophila". Journal of Bacteriology. 193 (5): 1114–1121. doi:10.1128/JB.01146-10. PMC 3067580. PMID 21169481.
- ^ Schewe MJ, Suzuki DT, Erasmus U (July 1971). "The genetic effects of mitomycin C in Drosophila melanogaster. II. Induced meiotic recombination". Mutation Research. 12 (3): 269–279. doi:10.1016/0027-5107(71)90015-7. PMID 5563942.
- ^ Bernstein H, Bernstein C, Michod RE (February 2012). "DNA repair as the primary adaptive function of sex in bacteria and eukaryotes.". In Kimura S, Shimizu S (eds.). DNA Repair: New Research. Hauppauge, N.Y.: Nova Sci. Publ. pp. 1–49. ISBN 978-1-62100-808-8.
External links
[edit]- Clinical trial number NCT02793128 for "The OLYMPUS Study - Optimized DeLivery of Mitomycin for Primary UTUC Study (Olympus)" at ClinicalTrials.gov
