FABP1
FABP1 is a human gene coding for the protein product FABP1 (Fatty Acid-Binding Protein 1). It is also frequently known as liver-type fatty acid-binding protein (LFABP).
FABP1 is primarily expressed in the liver where it is involved in the binding, transport and metabolism of long-chain fatty acids (LCFAs), endocannabinoids, phytocannabinoids (and less so for synthetic cannabinoid receptor (CBR) agonists and antagonists) and other hydrophobic molecules.[5][6][7][8] Altered expression of the protein has been linked to metabolic conditions including obesity.[9]
Discovery
[edit]The fatty acid-binding proteins (FABPs) were initially discovered in 1972 with experiments using 14C labelled oleate to identify the presence of a soluble fatty acid carrier in the enterocyte responsible for intestinal absorption of (LCFAs).[10] Since then, ten members of the FABP family have been identified in the human genome. Nine are well established (FABP1-9) with a recently discovered tenth (FABP12).[7] Each FABP corresponds to particular organs/tissue around the body where they play a role in fatty-acid uptake, transport and metabolism.[10]
Gene location
[edit]The human FABP1 gene is located on the short (p) arm of chromosome 2 from base pair 88,122,982 to base pair 88,128,131.[11]
Protein structure
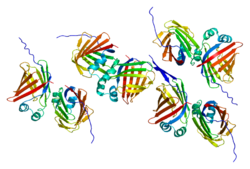
[edit]FABP1 has been found to have a unique structure compared to other members of the FABP family, allowing it to bind multiple ligands simultaneously.[12] It also has a larger solvent-accessible core compared to other FABPs allowing more diverse substrate binding.[7] The “portal hypothesis” has been proposed to explain the binding process of FABPs.[7] It has been suggested that fatty acids enter the solvent-accessible area of the protein through a dynamic region consisting of α-helix II and turns between the βC-βD and βE-βF loops.[13] The fatty acid is then bound in the protein cavity for transport.[13]
Function
[edit]The FABPs are a family of small, highly conserved cytoplasmic proteins involved in the binding of LCFAs. FABP1 is expressed abundantly in the human liver where it accounts for 7-11% of the total cytosolic protein, and can also be found in the intestine, kidney, pancreas, stomach and lung.[7][14] FABP1 is unique in the wider range of other hydrophobic ligands it can bind including bilirubin, monoglycerides, bile acids and fatty acyl CoA.[15][16][17][18] It has been proposed that FABP1 plays a significant role in preventing cytotoxicity by binding heme, fatty acids and other molecules that are potentially toxic when unbound.[12]
Mutations
[edit]A missense mutation within exon 3 of the human FABP1 gene results in a Thr to Ala substitution, T94A, in the protein product.[19] Carriers of this particular single nucleotide polymorphism (SNP) exhibit higher baseline plasma-free fatty acid levels, lower BMI and a smaller waist circumference.[19] The T94A mutant has also been associated with metabolic syndrome conditions, cardiovascular disease and T2DM.[19]
Protein expression
[edit]Suppression
[edit]Studies with mice to determine the effect of suppressing their Fabp1 gene ortholog have been performed. When provided with high-fat or high-cholesterol based diets those with suppressed FABP1 expression demonstrated a significant impact on metabolic regulation and weight gain.[20][21][22][23][24]
Increased levels in obesity
[edit]A study in Chinese young adults indicates a strong relationship between serum FABP1 levels and lipid profile, body measurements and homeostatic parameters.[9] Increased BMI and insulin resistance in subjects demonstrated higher serum FABP1 with a particular correlation in subjects with central adiposity.[9] This elevation is suggested to occur as a compensatory up-regulation of the protein in an attempt to counter the high metabolic stress associated with obesity. Alternately obesity may in fact lead the human body to develop resistance to the actions of FABP1 leading to the compensatory up-regulation.[9]
Disease marker
[edit]Evaluation of increased levels of urinary and serum FABP1 have also shown to be effective markers in the detection of intestinal ischaemia, progressive end-stage renal failure and ischaemic damage caused by renal transplantation or cardiac bypass surgery.[25][26][27]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000163586 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000054422 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Schroeder F, McIntosh AL, Martin GG, Huang H, Landrock D, Chung S, Landrock KK, Dangott LJ, Li S, Kaczocha M, Murphy EJ, Atshaves BP, Kier AB (June 2016). "Fatty Acid Binding Protein-1 (FABP1) and the Human FABP1 T94A Variant: Roles in the Endocannabinoid System and Dyslipidemias". Lipids. 51 (6): 655–76. doi:10.1007/s11745-016-4155-8. PMC 5408584. PMID 27117865.
- ^ Huang H, McIntosh AL, Martin GG, Landrock D, Chung S, Landrock KK, Dangott LJ, Li S, Kier AB, Schroeder F (September 2016). "FABP1: A Novel Hepatic Endocannabinoid and Cannabinoid Binding Protein". Biochemistry. 55 (37): 5243–55. doi:10.1021/acs.biochem.6b00446. PMC 5322802. PMID 27552286.
- ^ a b c d e Smathers RL, Petersen DR (March 2011). "The human fatty acid-binding protein family: evolutionary divergences and functions". Human Genomics. 5 (3): 170–91. doi:10.1186/1479-7364-5-3-170. PMC 3500171. PMID 21504868.
- ^ Huang, Huan; McIntosh, Avery L.; Martin, Gregory G.; Landrock, Danilo; Chung, Sarah; Landrock, Kerstin K.; Dangott, Lawrence J.; Li, Shengrong; Kier, Ann B. (2016-09-20). "FABP1: A Novel Hepatic Endocannabinoid and Cannabinoid Binding Protein". Biochemistry. 55 (37): 5243–5255. doi:10.1021/acs.biochem.6b00446. ISSN 1520-4995. PMC 5322802. PMID 27552286.
- ^ a b c d Shi J, Zhang Y, Gu W, Cui B, Xu M, Yan Q, Wang W, Ning G, Hong J (2012-11-07). "Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults". PLOS ONE. 7 (11): e48777. Bibcode:2012PLoSO...748777S. doi:10.1371/journal.pone.0048777. PMC 3492433. PMID 23144966.
- ^ a b Ockner RK (1990). "Historic overview of studies on fatty acid-binding proteins". Molecular and Cellular Biochemistry. 98 (1–2): 3–9. doi:10.1007/bf00231361. PMID 2266967. S2CID 29623496.
- ^ "FABP 1". Gene Cards. Retrieved 2016-10-16.
- ^ a b Wang G, Bonkovsky HL, de Lemos A, Burczynski FJ (December 2015). "Recent insights into the biological functions of liver fatty acid binding protein 1". Journal of Lipid Research. 56 (12): 2238–47. doi:10.1194/jlr.R056705. PMC 4655993. PMID 26443794.
- ^ a b Sacchettini JC, Gordon JI, Banaszak LJ (July 1989). "Crystal structure of rat intestinal fatty-acid-binding protein. Refinement and analysis of the Escherichia coli-derived protein with bound palmitate". Journal of Molecular Biology. 208 (2): 327–39. doi:10.1016/0022-2836(89)90392-6. PMID 2671390.
- ^ Vergani L, Fanin M, Martinuzzi A, Galassi A, Appi A, Carrozzo R, Rosa M, Angelini C (1990). "Liver fatty acid-binding protein in two cases of human lipid storage". Molecular and Cellular Biochemistry. 98 (1–2): 225–30. doi:10.1007/bf00231388. PMID 2266963. S2CID 25712825.
- ^ Levi AJ, Gatmaitan Z, Arias IM (November 1969). "Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions". Journal of Clinical Investigation. 48 (11): 2156–67. doi:10.1172/jci106182. PMC 297469. PMID 4980931.
- ^ Storch J (1993). "Diversity of fatty acid-binding protein structure and function: studies with fluorescent ligands". Molecular and Cellular Biochemistry. 123 (1–2): 45–53. doi:10.1007/BF01076474. PMID 8232268. S2CID 11631346.
- ^ Thumser AE, Wilton DC (December 1996). "The binding of cholesterol and bile salts to recombinant rat liver fatty acid-binding protein". Biochemical Journal. 320 (3): 729–33. doi:10.1042/bj3200729. PMC 1217991. PMID 9003356.
- ^ Mishkin S, Turcotte R (April 1974). "The binding of long chain fatty acid CoA to Z, a cytoplasmic protein present in liver and other tissues of the rat". Biochemical and Biophysical Research Communications. 57 (3): 918–26. doi:10.1016/0006-291X(74)90633-0. PMID 4827841.
- ^ a b c Brouillette C, Bossé Y, Pérusse L, Gaudet D, Vohl MC (2004). "Effect of liver fatty acid binding protein (FABP) T94A missense mutation on plasma lipoprotein responsiveness to treatment with fenofibrate". Journal of Human Genetics. 49 (8): 424–32. doi:10.1007/s10038-004-0171-2. PMID 15249972.
- ^ Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F (November 2010). "Liver fatty acid-binding protein and obesity". The Journal of Nutritional Biochemistry. 21 (11): 1015–32. doi:10.1016/j.jnutbio.2010.01.005. PMC 2939181. PMID 20537520.
- ^ Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F (January 2006). "Liver fatty acid binding protein gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice". American Journal of Physiology. Gastrointestinal and Liver Physiology. 290 (1): G36-48. doi:10.1152/ajpgi.00510.2004. PMID 16123197.
- ^ Atshaves BP, McIntosh AL, Storey SM, Landrock KK, Kier AB, Schroeder F (February 2010). "High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene-ablated mice". Lipids. 45 (2): 97–110. doi:10.1007/s11745-009-3379-2. PMC 2831749. PMID 20035485.
- ^ Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO (December 2003). "Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene". Journal of Biological Chemistry. 278 (51): 51664–72. doi:10.1074/jbc.M309377200. PMID 14534295.
- ^ "Fabp1 fatty acid binding protein 1, liver [ Mus musculus (house mouse) ]". Gene. National Library of Medicine, National Center for Biotechnology Information. 9 May 2024. Gene ID #14080. Retrieved 5 August 2024.
- ^ Thuijls G, van Wijck K, Grootjans J, Derikx JP, van Bijnen AA, Heineman E, Dejong CH, Buurman WA, Poeze M (February 2011). "Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins". Annals of Surgery. 253 (2): 303–8. doi:10.1097/sla.0b013e318207a767. PMID 21245670. S2CID 206078242.
- ^ Obata Y, Kamijo-Ikemori A, Ichikawa D, Sugaya T, Kimura K, Shibagaki Y, Tateda T (February 2016). "Clinical usefulness of urinary liver-type fatty-acid-binding protein as a perioperative marker of acute kidney injury in patients undergoing endovascular or open-abdominal aortic aneurysm repair". Journal of Anesthesia. 30 (1): 89–99. doi:10.1007/s00540-015-2095-8. PMC 4750552. PMID 26585768.
- ^ Kamijo A, Sugaya T, Hikawa A, Kimura K (March 2003). "[Urinary fatty acid binding protein as a new clinical marker for the progression of chronic renal disease]". Rinsho Byori. 51 (3): 219–24. doi:10.1016/j.lab.2003.08.001. PMID 12707994.
Further reading
[edit]- Glatz JF, Börchers T, Spener F, van der Vusse GJ (1995). "Fatty acids in cell signalling: modulation by lipid binding proteins". Prostaglandins, Leukotrienes, and Essential Fatty Acids. 52 (2–3): 121–7. doi:10.1016/0952-3278(95)90010-1. PMID 7784447.
- Kaikaus RM, Chan WK, Ortiz de Montellano PR, Bass NM (1993). "Mechanisms of regulation of liver fatty acid-binding protein". Molecular and Cellular Biochemistry. 123 (1–2): 93–100. doi:10.1007/BF01076479. PMID 8232272. S2CID 11639043.
- Londraville RL (June 1996). "Intracellular fatty acid-binding proteins: putting lower vertebrates in perspective". Brazilian Journal of Medical and Biological Research. 29 (6): 707–20. PMID 9070383.
- Börchers T, Hohoff C, Buhlmann C, Spener F (July 1997). "Heart-type fatty acid binding protein - involvement in growth inhibition and differentiation". Prostaglandins, Leukotrienes, and Essential Fatty Acids. 57 (1): 77–84. doi:10.1016/S0952-3278(97)90496-8. PMID 9250612.
- Carroll SL, Roth KA, Gordon JI (December 1990). "Liver fatty acid-binding protein: a marker for studying cellular differentiation in gut epithelial neoplasms". Gastroenterology. 99 (6): 1727–35. doi:10.1016/0016-5085(90)90480-o. PMID 1699834.
- Sweetser DA, Birkenmeier EH, Klisak IJ, Zollman S, Sparkes RS, Mohandas T, Lusis AJ, Gordon JI (November 1987). "The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships". Journal of Biological Chemistry. 262 (33): 16060–71. doi:10.1016/S0021-9258(18)47696-X. PMID 2824476.
- Chan L, Wei CF, Li WH, Yang CY, Ratner P, Pownall H, Gotto AM, Smith LC (March 1985). "Human liver fatty acid binding protein cDNA and amino acid sequence. Functional and evolutionary implications". Journal of Biological Chemistry. 260 (5): 2629–32. doi:10.1016/S0021-9258(18)89406-6. PMID 3838309.
- Lowe JB, Boguski MS, Sweetser DA, Elshourbagy NA, Taylor JM, Gordon JI (March 1985). "Human liver fatty acid binding protein. Isolation of a full length cDNA and comparative sequence analyses of orthologous and paralogous proteins". Journal of Biological Chemistry. 260 (6): 3413–7. doi:10.1016/S0021-9258(19)83637-2. PMID 3838313.
- Murphy EJ (August 1998). "L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L cells". American Journal of Physiology. 275 (2 Pt 1): G244-9. doi:10.1152/ajpgi.1998.275.2.G244. PMID 9688651.
- Wolfrum C, Börchers T, Sacchettini JC, Spener F (February 2000). "Binding of fatty acids and peroxisome proliferators to orthologous fatty acid binding proteins from human, murine, and bovine liver". Biochemistry. 39 (6): 1469–74. doi:10.1021/bi991638u. PMID 10684629.
- Wolfrum C, Borrmann CM, Borchers T, Spener F (February 2001). "Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus". Proceedings of the National Academy of Sciences of the United States of America. 98 (5): 2323–8. Bibcode:2001PNAS...98.2323W. doi:10.1073/pnas.051619898. PMC 30137. PMID 11226238.
- Schroeder F, Atshaves BP, Starodub O, Boedeker AL, Smith RR, Roths JB, Foxworth WB, Kier AB (March 2001). "Expression of liver fatty acid binding protein alters growth and differentiation of embryonic stem cells". Molecular and Cellular Biochemistry. 219 (1–2): 127–38. doi:10.1023/A:1010851130136. PMID 11354243. S2CID 37941511.
- Zimmerman AW, van Moerkerk HT, Veerkamp JH (September 2001). "Ligand specificity and conformational stability of human fatty acid-binding proteins". The International Journal of Biochemistry & Cell Biology. 33 (9): 865–76. doi:10.1016/S1357-2725(01)00070-X. PMID 11461829.
- Mésange F, Sebbar M, Capdevielle J, Guillemot JC, Ferrara P, Bayard F, Poirot M, Faye JC (2003). "Identification of two tamoxifen target proteins by photolabeling with 4-(2-morpholinoethoxy)benzophenone". Bioconjugate Chemistry. 13 (4): 766–72. doi:10.1021/bc015588t. PMID 12121132.