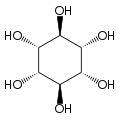
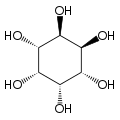
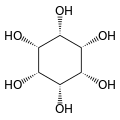
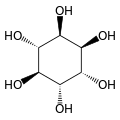
Cyclohexane-1,2,3,4,5,6-hexol
 cis-Inositol
| |
| Names | |
|---|---|
| IUPAC name
cyclohexane-1,2,3,4,5,6-hexol
| |
| Systematic IUPAC name
Cyclohexane-1,2,3,4,5,6-hexol | |
| Other names
Inositol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cyclohexane-1,2,3,4,5,6-hexol is a family of chemical compounds with formula C6H12O6, whose molecule consists of a ring of six carbon atoms, each bound to one hydrogen atom and one hydroxyl group (–OH). There are nine stereoisomers, that differ by the position of the hydroxyl groups relative to the mean plane of the ring. All these compounds are sometimes called inositol, although this name (especially in biochemistry and related sciences) most often refers to a particular isomer, myo-inositol, which has many important physiological roles and medical uses.
These compounds are classified as sugars, specifically carbocyclic sugars or sugar alcohols, to distinguish them from the more common aldoses like glucose. They generally have sweet taste.[2]
These compounds form several esters with biochemical and industrial importance, such as phytic acid and phosphatidylinositol phosphate,
Isomers and structure
[edit]The nine stereoisomers of cyclohexane-1,2,3,4,5,6-hexol are distinguished by prefixes: myo-, scyllo-, muco-, D-chiro-, L-chiro-, neo-, allo-, epi-, and cis-inositol.
As their names indicate, L- and D-chiro inositol are chiral, a pair enantiomers (mirror-image forms). All the others are meso compounds (indistinguishable from their mirror images).[3]
Racemate
[edit]The designation rac-chiro-inositol has been used for the racemic mixture (racemate) of equal parts of the two chiro isomers. It crystallizes as a single phase, rather than separate D and L crystals, that melts at 250 °C (which is 4–5 °C higher than the melting point of the pure enantiomers) and decomposes between 308 and 344 °C. The crystal structure is monoclinic with the group. The crystal cell parameters are a = 1014.35 pm, b = 815.42 pm, c = 862.39 pm, β = 92.3556°, Z = 4. The cell volume is 0.71270 nm3, or about 0.178 nm3 per molecule (which is a bit smaller than the typical volumes of other isomers).[4]
Ring conformation
[edit]As in cyclohexane, the C6 ring of these compounds can be in two conformations, "boat" and "chair". The relative stability of the two forms varies with the isomer, generally favoring the conformation where the hydroxyls are farthest apart from each other.[5]
Melting points
[edit]Some of the stereoisomers crystallize in more than one polymorph, with different densities and melting points — which range from 225 °C for myo-inositol to about 360 °C for polymorph "B" of scyllo-inositol.[4] There is a clear correlation between the melting points and the number and type of chains of hydrogen-bonded hydroxyls.[6]
Biochemistry
[edit]All isomers except allo- and cis- occur in nature, although myo-inositol is substantially more abundant and important than the others.[7][8]
In humans, myo-inositol is synthesized mostly in the kidneys, from glucose 6-phosphate.[9] Small amounts of myo-inositol are then converted by a specific epimerase to D-chiro-inositol,[10] which is an important messenger molecule in insulin signaling.[11]
A 2020 study found detectable amounts of epi-, neo-, chiro-, scyllo-, and myo-inositol in the urine of women, pregnant or not. Concentrations of myo and scyllo increased significantly in the third trimester of pregnancy, with scyllo varying between 20% and 40% of myo. Concentrations of epi, neo, and chiro were always a few percent of those of myo, except that chiro- reached 20% of myo in the second trimester of pregnancy.[8]
The bacterium Bacillus subtilis can metabolize myo-, scyllo-, and D-chiro-inositol.and convert to and from these three isomers.[12]
Phytic acids
[edit]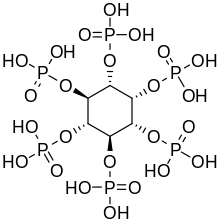
Plants synthesize inositol hexakis-dihydrogenphosphate, also called phytic acid or IP6, as a storage of phosphorus[13] Inositol penta- (IP5), tetra- (IP4), and triphosphate (IP3) are also called "phytates"
References
[edit]- ^ Merck Index (11th ed.). p. 4883.
- ^ G. G. Birch and M. G. Lindley (1973): "Cyclohexane polyols as sweet analogues of the sugars". Journal of Food Science, volume 38, issue 7, pages 1179-1181. doi:10.1111/j.1365-2621.1973.tb07232.x
- ^ Majumder, A. L.; Biswas, B. B. (2006-10-03). Biology of Inositols and Phosphoinositides. Springer Science & Business Media. ISBN 9780387276007.
- ^ a b Sándor L. Bekö, Edith Alig, Martin U. Schmidt, Jacco van de Streek (2014): "On the correlation between hydrogen bonding and melting points in the inositols". International Union of Crystallography Journal (IUCrJ), volume 1, part 1, pages 61-73. doi:10.1107/S2052252513026511
- ^ Brady, S.; Siegel, G.; Albers, R. W.; Price, D. (2005). Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Academic Press. p. 348. ISBN 9780080472072.
- ^ Alexandra Simperler, Stephen W. Watt, P. Arnaud Bonnet, William Jones, W. D. Samuel Motherwell (2006): "Correlation of melting points of inositols with hydrogen bonding patterns". CrystEngComm, volume 8, pages 589-600 doi:10.1039/B606107A
- ^ William R. Sherman, Mark A. Stewart, Mary M. Kurien, Sally L. Goodwin (1968): "The measurement of myo-inositol, myo-inosose-2 and scyllo-inositol in mammalian tissues". Biochimica et Biophysica Acta (BBA), volume 158, issue 2, pages 197-205 doi:10.1016/0304-4165(68)90131-1
- ^ a b Irina Monnard, Thierry Bénet, Rosemarie Jenni, Sean Austin, Irma Silva-Zolezzi, Jean-Philippe Godin (2020): "Plasma and urinary inositol isomer profiles measured by UHPLC-MS/MS reveal differences in scyllo-inositol levels between non-pregnant and pregnant women". Analytical and Bioanalytical Chemistry, volume 412, pages 7871–7880. doi:10.1007/s00216-020-02919-8
- ^ Parthasarathy, L. K.; Seelan, R. S.; Tobias, C.; Casanova, M. F.; Parthasarathy, R. N. (2006). Mammalian inositol 3-phosphate synthase: its role in the biosynthesis of brain inositol and its clinical use as a psychoactive agent. Subcellular Biochemistry. Vol. 39. pp. 293–314. doi:10.1007/0-387-27600-9_12. ISBN 978-0-387-27599-4. PMID 17121280.
- ^ Kiani AK, Paolacci S, Bertelli M (2021). "From Myo-inositol to D-chiro-inositol molecular pathways". European Review for Medical and Pharmacological Sciences. 25 (5): 2390–2402. doi:10.26355/eurrev_202103_25279. PMID 33755975.
- ^ Tabrizi R, Ostadmohammadi V, Asemi Z (2018). "The effects of inositol supplementation on lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials". Lipids in Health and Disease. 17 (1): 123. doi:10.1186/s12944-018-0779-4. PMC 5968598. PMID 29793496.
- ^ Masaru Yamaoka, Shin Osawa, Tetsuro Morinaga, Shinji Takenaka, Ken-ichi Yoshida (2011): "A cell factory of Bacillus subtilis engineered for the simple bioconversion of myo-inositol to scyllo-inositol, a potential therapeutic agent for Alzheimer's disease". Microbial Cell Factories, volume 10, article number 69. doi:10.1186/1475-2859-10-69
- ^ "Phytic acid". www.phytochemicals.info. Archived from the original on 7 March 2018. Retrieved 2018-05-02.
{{cite web}}: CS1 maint: unfit URL (link)